CV-19 fears
CV-19 fears and market conditions
Coronavirus fear
-
- 01/06/2020 – seems like vaccine roll out is accelerating. Hope it can be faster
- U.S. health experts continue to raise alarm over the country’s slow vaccine rollout. As of Tuesday morning, just 4.8 million vaccine doses had been administered across the country, out of a total 17 million doses distributed to administration sites, according to the CDC. CVS Health said Wednesday it’s on pace to complete the first round of vaccinations at long-term care facilities by Jan. 25. The company also said a small number of vaccine doses could be made available at pharmacy locations in the coming weeks ahead of a broad rollout. Experts have recommended a number of changes to improve the pace of vaccinations, including broadening early eligibility and reducing stored capacity for second doses.
- 01/05/2021 – be very cautious about the new variance of virus. Quickly get vaccine to everyone is very critical to fight the virus. Operation Warp Speed’s director said on Dec. 3 that everyone who wants a vaccine will be able to get one by June. Projections are currently falling short, however.
South African Covid variant appears to ‘obviate’ antibody drugs, Dr. Scott Gottlieb says
- “The South Africa variant is very concerning right now because it does appear that it may obviate some of our medical countermeasures, particularly the antibody drugs,” Dr. Scott Gottlieb said.
- The South African variant is also known as 501.V2, and in mid-December officials reported that 501.V2 had been largely replacing other strains of the coronavirus as early as November.
- More than 17 million Covid doses have been distributed to states, but only 4.8 million Americans have received their first shot according to the Centers for Disease Control and Prevention.
Dr. Scott Gottlieb warned that vaccinating Americans against Covid is more critical than ever, especially as the new South Africa variant appears to inhibit antibody drugs.
“The South Africa variant is very concerning right now because it does appear that it may obviate some of our medical countermeasures, particularly the antibody drugs,” said the former FDA chief in the Trump administration in an interview on CNBC’s “The News with Shepard Smith” on Tuesday evening. “Right now that strain does appear to be prevalent in South America and Brazil, the two parts of the world, right now, that are in their summer, but also experiencing a very dense epidemic, and that’s concerning.
The South African variant is also known as 501.V2, and in mid-December officials reported that 501.V2 had been largely replacing other strains of the coronavirus as early as November. South Africa has already sustained the more than 1.1 million COVID-19 cases and more than 30,000 deaths, the most on the African continent.
Gottlieb cited experimental evidence from Bloom Lab, and explained 501.V2 does appear to partially escape prior immunity. It means that some of the antibodies people produce when they get infected with Covid, as well as the antibody drugs, may not be quite as effective.
“The new variant has mutated a part of the spike protein that our antibodies bind to, to try to clear the virus itself, so this is concerning,” Gottlieb said. “Now, the vaccine can become a backstop against these variants really getting more of a foothold here in the United States, but we need to quicken the pace of vaccination.”
Operation Warp Speed’s director of supply production and distribution Ret. Lt. Gen. Paul Ostrowski told host Shepard Smith on Dec. 3 that everyone who wants a vaccine will be able to get one by June. Projections are currently falling short, however. More than 17 million Covid doses have been distributed to states, but only 4.8 million Americans have received their first shot according to the Centers for Disease Control and Prevention.
Gottlieb suggested working through prioritized categories of people more quickly, expanding the number of vaccination sites, and stockpiling a smaller percentage of dosages in order to vaccinate more Americans.
“It really is a race against time trying to get more vaccine into people’s arms before these new variants become more prevalent here in the United States,” said Gottlieb.
-
- 12/30/2020 – this vaccine can be a real game changer since it is cheap and easy to mass produce and mass transportation. But it might only be available to US and Europe until February 2021. Also, China is rolloing out vaccine for 50 millions people before Lunar festival. In addition, Regeneron Pharmaceuticals Inc. said a trial found its therapy drug effective and that the study would continue.
Covid-19 Vaccine Made by AstraZeneca, Oxford Is Authorized by U.K.
Green light for emergency use comes as Britain grapples with a surge in cases and a new variant
“The Oxford-AstraZeneca vaccine will lead to a significant increase in vaccination as it is cheap and easy to mass produce. Crucially it can be stored in a standard fridge – unlike the Pfizer-BioNTech jab which needs ultra cold storage at -70C – so it will be far easier to get the Oxford vaccine to care homes and GP surgeries.” — BBC
AstraZeneca said Wednesday it will supply millions of doses in the first quarter of next year, without specifying an exact number. The U.K. has ordered up to 100 million doses of the vaccine, enough for 50 million people.
It is unclear how quickly other nations might authorize the vaccine. A large clinical trial is under way in the U.S., where AstraZeneca executives expect to have full trial data to submit to U.S. regulators by February, according to a person familiar with the matter. They have been submitting batches of data to European regulators and are poised for potential authorization across the European Union by February, the person said.
How Viral Vector Vaccines Work
AstraZeneca’s new vaccine relies on a different mechanism for conferring immunity than traditional vaccines.
NEW ENGLAND JOURNAL OF MEDICINE PUBLISHES POSITIVE INITIAL REGENERON ANTIBODY COCKTAIL RESULTS IN NON-HOSPITALIZED PATIENTS WITH COVID-19
Chinese Covid-19 Vaccine Is 79% Effective, Interim Phase 3 Results Show
Candidate’s maker seeks conditional approval from Chinese regulators
China plans to inoculate as many as 50 million people ahead of the Lunar New Year holiday in February with Sinopharm and Sinovac vaccines, people familiar with the matter said earlier this month.
Some Chinese developers, including Sinopharm and Sinovac, have said their vaccines can be stored above freezing, making them easier to transport and store in less-developed countries. The Pfizer-BioNTech shots must be stored at ultralow temperatures, requiring specialized equipment, while Moderna’s are kept around minus 4 degrees Fahrenheit.
Regeneron’s Antibodies Appear to Help Hospitalized Patients—and Could Help 2021 Revenue
As everyone waits for Covid-19 vaccine shots, cases have surged and a new variant of the coronavirus that’s more infectious has been discovered. That’s why it was encouraging when Regeneron Pharmaceuticals said late Tuesday that its REGN-COV2 cocktail of antibodies against the virus seems to be helping hospitalized patients in a large clinical trial. If the final readout is good, the treatment could help manage the Covid upsurge and bring Regeneron a 2021 revenue boost.
Infusions of synthetic antibodies from Regeneron and Eli Lilly (LLY) each got emergency authorizations from U.S. regulators for use in keeping infected patients out of the hospital. But those treatments haven’t yet proven their benefit in sicker patients already hospitalized. In October, Lilly stopped recruitment for a trial of its single antibody bamlanivimab in hospitalized Covid patients after a monitoring committee concluded the treatment wasn’t working. Regeneron’s Tuesday announcement that its data monitors decided to continue its own trial is positive news.
When patients without natural antibodies got Regeneron’s cocktail, their virus levels dropped dramatically and their rates of death or need for mechanical ventilation fell by 22% compared with those who received a placebo, according to preliminary data in the press release. Benefits were particularly strong in the first week after hospitalization. The data are preliminary, cautioned Yancopoulos, and trials of the Regeneron antibodies will continue in the U.S. and the U.K.
“This outcome represents a middle-of-the-road result with neither early discontinuation for overwhelming success (a best case result) nor early stoppage for futility (worst case result),” wrote RBC Capital Markets analyst Kennen MacKay, in a Wednesday note. While seeing Tuesday’s news as “incrementally positive” for Regeneron, MacKay rates the stock at Sector Perform.
-
- 12/29/2020 – factors to drive market high
Favorable developments on several fronts, including the relief package, vaccine distribution, the Federal Reserve’s extra efforts to bolster the economy, and the resolution of Brexit terms, kept stocks buoyant.
-
- 12/29/2020 – this might be good for RTX and oil industry
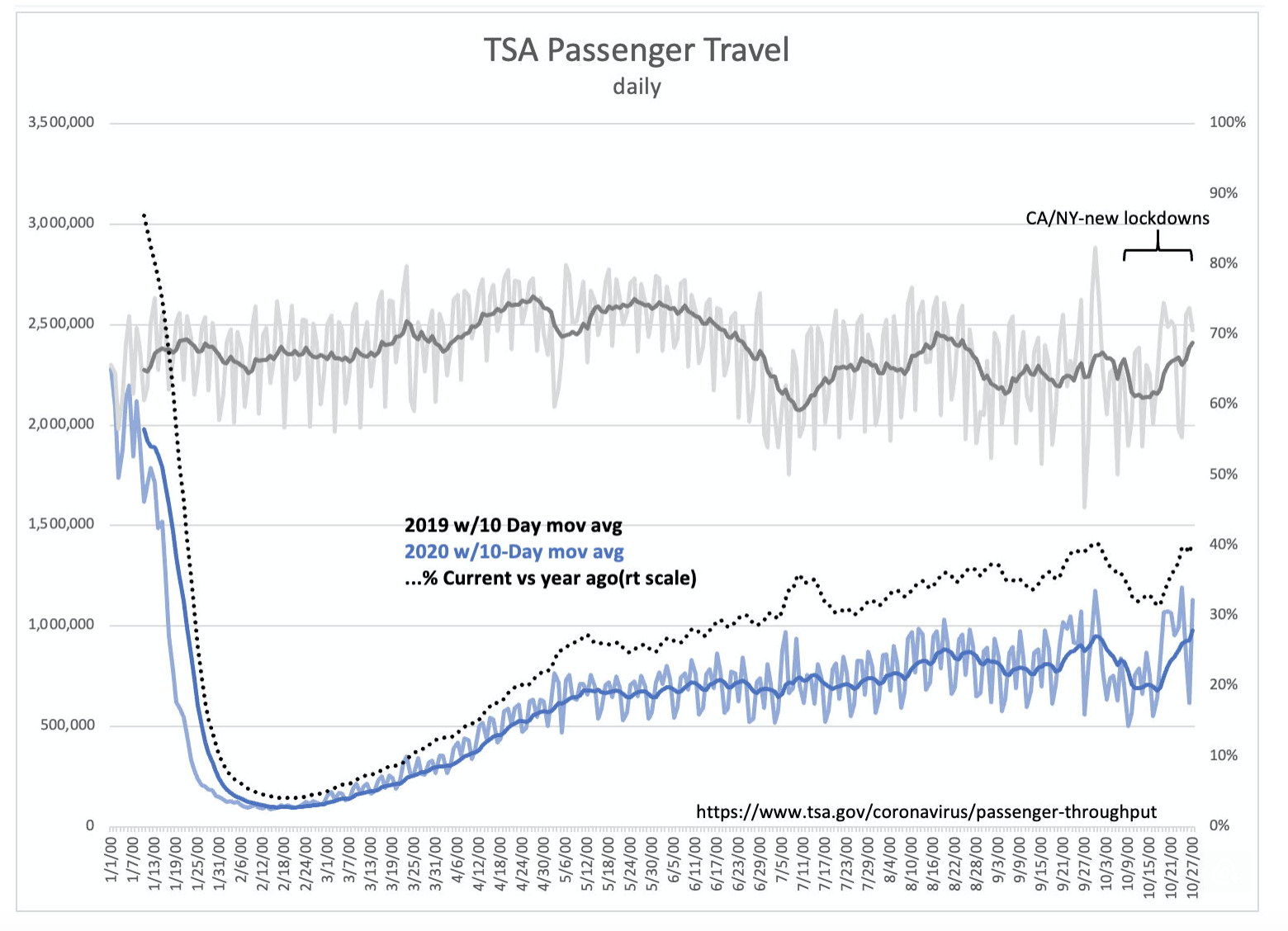
“Davidson” submits:
After % pull back from 2019, TSA Travelers post new high vs 2019 of 40.6%-higher than Thanksgiving’s peak. New lockdowns initially seemed to have an impact driving year ago comparisons to low 30 are now no longer reflected in this data.
-
- 12/27/2020 – this sounds like a great idea to roll out vacine faster, and turn around economy faster
Possibility of one-dose vaccine raises hopes for faster rollout
The first coronavirus vaccine shots administered across the country have raised hopes for a breakthrough in the fight against COVID-19, but experts are now raising an even more hopeful possibility: that people might only need one shot instead of the current two-dose regimen.
The prospect would effectively double the number of vaccine doses available and allow more people to be vaccinated quickly. But the idea has set off a debate, with experts saying there isn’t enough evidence yet to justify a single dose and people should plan to get two doses.
The push in favor of exploring the idea of a single-dose vaccine crystallized with a recent New York Times op-ed from Michael Mina, an immunologist at the Harvard T.H. Chan School of Public Health, and Zeynep Tufecki, a sociologist who has written extensively on the pandemic.
They called for immediately starting a new clinical trial to study whether one dose of the vaccine is sufficient. They cited data from the trials already conducted for the Pfizer and Moderna vaccines that showed protection began after the first dose, with as much as around 90 percent efficacy, compared to around 95 percent efficacy after two doses.
-
- 12/21/2020 – vaccine might still be effective for the new covid strain, and eventually we need to update the vaccines. Market drops due to this bad news, so it might be a good time to buy low? The variant of covid strain might be already in US, but vaccine might still be effective
Dr. Scott Gottlieb says new UK Covid strain ‘will not slip past our vaccines very easily’
- A new strain of coronavirus circulating in the U.K. is believed to be more than 70% more transmissible than existing variants.
- However, Dr. Scott Gottlieb said existing Covid-19 vaccines are likely to provide protection against it.
- The virus mutation also does not appear to have made it more deadly, according to the former FDA chief.
- Dr. Scott Gottlieb told CNBC on Monday that existing Covid-19 vaccines will likely provide protection against the new coronavirus strain circulating in the U.K.British Prime Minister Boris Johnson on Saturday implemented strict lockdowns in London and large parts of southern England in response to rising coronavirus infections. He said the growth in cases appeared to be stemming from a new variant of the coronavirus that more than 70% more transmissible than existing strains.Gottlieb, a former U.S. Food and Drug Administration commissioner, said on “Squawk Box” that the evidence does suggest the new U.K. variant transmits more easily. But, he cautioned, “It doesn’t seem to have mutated the surface proteins of the virus in a way that they would slip past our vaccines or prior immunity. In fact, we don’t think that that’s the case.”Gottlieb, a board member of Pfizer, which makes one of two Covid-19 vaccines to receive limited FDA approval, has also said the U.K. virus strain does not seem to be more deadly than previous variants during the pandemic.The fact the coronavirus has mutated is not surprising, Gottlieb said. It is common for viruses to do so.“Some viruses like flu evolve their surface proteins very quickly, and that’s why we need a different flu vaccine every season,” said Gottlieb, who led the FDA in the Trump administration from 2017 to 2019. “Some viruses can’t really change their surface proteins, like measles. This [coronavirus] seems to fall some place in between. It’s not going to change it’s surface proteins very rapidly, that spike protein, but it will change it over time.”The developments around the new U.K. virus strain come as vaccine rollout picks up steam in Britain and U.S. alike. On Friday, the FDA approved Moderna’s Covid-19 vaccine for emergency use, paving the way for distribution to begin this week.Gottlieb sought to assure people that the existing vaccines would be effective against the U.K. virus strain — which also has been detected in a patient in Italy — and by extension help curtail a pandemic that has infected almost 77 million people worldwide and killed nearly 1.7 million people, according to data compiled by Johns Hopkins University.“It’s probably a good thing that we used the entire spike protein in our vaccines because what we’re getting is what we call a polyclonal response. We’re developing antibodies to many different regions of that protein, so even if one part of that protein were mutated and some antibodies no longer recognize it, there would be antibodies to other parts of that protein,” Gottlieb explained. Antibodies help fight infections. “So this probably will not slip past our vaccines very easily, but eventually we will have to update the vaccines.”
Trump Covid vaccine czar says ‘extremely low’ chance Pfizer and Moderna shots won’t work against new strain
PUBLISHED MON, DEC 21 20201:36 PM ESTUPDATED MON, DEC 21 20203:26 PM EST
- Trump’s vaccine czar said Monday he expects Pfizer’s and Moderna’s Covid-19 shots will be effective against a new mutation of the virus found in the U.K.
- That country on Saturday said it identified a mutation that can spread more quickly than previous variants.
- Countries including Italy, Germany, Canada and Israel have barred flights from the U.K. following reports of the new strain.
- Dr. Scott Gottlieb warned that the highly contagious, new mutation of Covid-19 found in the United Kingdom “is already in the U.S.”
- Scientists in the U.K. suggested the Covid variant makes the virus 50% more transmissible. However, there’s currently no sign that it makes the disease worse.
- Gottlieb said that the current logistics for vaccine distribution are “good,” but that some challenges may come as the population getting the vaccine widens.
The new variant of Covid-19 is forcing parts of the United Kingdom back into lockdown. The government imposed the tightest restrictions in London as well as other areas in southeast England, and families are no longer able to gather during Christmas, as previously planned. In an interview on CNBC’s “The News with Shepard Smith” on Monday evening, Gottlieb explained that the new mutation is likely a result of selective pressure on the virus itself.
“As the virus continues to spread around the world, we’re going to start to see more of these variants, and that’s why it’s important to get the population vaccinated and snuff out these infections,” Gottlieb said. “The more infections you have, the more chances that these variants start to propagate.”
Scientists in the U.K. suggested the Covid variant makes the virus 50% more transmissible, however, there’s currently no sign that it makes the disease worse. Both Eli Lilly and Regeneron, which make the antibody drugs to treat Covid, said their medicines should be effective against the variant. According to Reuters, BioNTech Chief Executive Ugur Sahin said his company would investigate the mutation, but looked at the situation with “with a degree of soberness.” BioNTech is Pfizer’s partner on the Covid vaccine. Gottlieb explained to host Shep Smith why he thinks vaccines will need to eventually adapt.
“The question is, is this virus going to change the surface proteins in a way that can obviate either the vaccines or prior immunity, and there’s no indication that it’s doing that right now, but over time it will evolve in ways where it can probably obviate prior infection or vaccines to some degree, so we’ll probably need to adapt our vaccines over time,” Gottlieb said.
-
- 12/20/2020 – interesting to know this. Noticed that US has 1B doses, which could be enough to cover all population. also notice that China is not on the map.

- 12/19/2020 – Senate has reached the deal. vote will come on Sunday.
Republicans, Democrats Reach Agreement Clearing Way For Virus Relief Votes
Vote on $900 billion package expected Sunday
WASHINGTON—Senate Democrats and Republicans have reached an agreement over constraints to the Federal Reserve’s emergency lending powers, clearing a last remaining hurdle for a $900 billion pandemic aid package. The compromise wouldn’t prevent the central bank from creating similar programs in the future, according to aides from both parties.
Senators reach deal on Fed powers, setting stage for coronavirus relief passage
Senate Minority Leader Charles Schumer (D-N.Y.) and Sen. Pat Toomey (R-Pa.) have reached agreement on language to curtail the Federal Reserve’s special lending authorities, setting the stage for passage of a coronavirus relief deal and omnibus spending package as early as Sunday.
At around 9 p.m. Republicans sent word to Schumer that they would accept language resolving the dispute over the Federal Reserve’s lending facilities, according to GOP aides.
The compromise will sweep out the $429 billion in unspent CARES Act funding for the Federal Reserve’s credit lending facilities and repurpose it as an offset for a new $900 billion coronavirus relief bill, GOP sources said.
- 12/19/2020 – Due date of negotiation on stimulus deal is 12:01 a.m. ET Monday morning. Watch out the outcome
As the U.S. races to deliver more life saving vaccines after Moderna’s two-dose shot was approved for emergency use, Congress is up against the clock to deliver economic lifelines for Americans who are struggling from the pandemic’s economic fallout.
For months, negotiations in Congress have failed to produce a new Covid stimulus deal, and this week was no different, even though they tied the talks to broader government funding legislation in effort to create pressure for a compromise.
That plan, however, has not worked so far. Lawmakers had to pass last-minute emergency funding on Friday to avert a government shutdown and create time for another round negotiations over the weekend.
If lawmakers do not reached a deal by 12:01 a.m. ET Monday morning, the government will shut down. Now, the negotiations are hung up over the Federal Reserve’s emergency lending powers.
- 12/17/2020 – the new cv-19 strain may not be that deadly and might not alter vaccine performance (preliminary results) – so far it is not that bad
What We Know About the New Covid-19 Strain in England
Variant is believed to spread 70% faster than earlier versions of the virus
Three main questions are now being investigated: Is the new variant more contagious, is it more likely to be fatal or cause serious illness, and is it more likely to defeat the body’s immune responses, including those encouraged by vaccines?
The provisional answers to those questions, as outlined by British scientific advisers on Saturday, are yes, no and no.
Patrick Vallance, the British government’s chief scientific adviser, said on Saturday that three types of study—of the virus’s genetic makeup, statistics and in the laboratory—have come together to show that this variant is significantly more prone to be transmitted among people than earlier strains.
Mr. Vallance said the conclusions that the mutation was less dangerous and unlikely to compromise the effectiveness of vaccines were preliminary. He said there were theoretical reasons why the new variant might alter the immune response, though there was no evidence so far that was the case.
“The working assumption is that the vaccine response should be adequate for this virus, but we need to keep vigilant about this,” he said.
According to Dr. Sanjana of the New York Genome Center, “Single mutations will generally not alter vaccine performance.”
British officials said they had no evidence the mutation was present abroad, though scientists say a similar mutation has appeared independently in South Africa.
- 12/16/2020 – will the deal come on this Friday?
Lawmakers Near Covid-19 Relief Deal With Second Round of Stimulus Checks
Roughly $900 billion package features payments to households, $300 a week in enhanced unemployment insurance, among other measures
WASHINGTON—Congressional leaders closed in on a roughly $900 billion coronavirus relief deal that includes another round of direct payments to households, according to lawmakers who aimed to pass the aid package before the week’s end.
After months of gridlock, the emerging agreement represented a breakthrough at a critical time in the pandemic, with distribution of a vaccine under way but hospitalizations hitting record highs and a new round of business restrictions weighing on the economy.
The package under discussion was expected to include, along with direct checks, $300 a week in enhanced unemployment insurance, funding for vaccine distribution, schools, small businesses and health-care providers, rental assistance and other relief measures. Its size, at just under $900 billion, marked a compromise between the two parties’ stances: more than the roughly $500 billion Republicans had backed and less than the $2.4 trillion bill Democrats passed in the House earlier this year.
“We’re still talking and I think we’re going to get there,” Senate Majority Leader Mitch McConnell (R., Ky.) said Wednesday afternoon.
- 12/13/2020 – Revised deal to split the stimulus plan packages into two proposals. Will this work?
Bipartisan congressional group splits Covid-19 relief package into two proposals
Washington (CNN)The bipartisan group of House and Senate negotiators that has struggled to finalize a $908 billion Covid relief package has made a decision: The lawmakers plan to split the package into two separate proposals. One bill will be a $748 billion proposal, which includes money for small business loans, jobless benefits and vaccine distribution — among other matters.
The other: A package that includes $160 billion for state and local aid that includes liability protections for businesses and other entities — the two biggest sticking points.
It’s uncertain how many senators and House members from the group will sign onto the second proposal, but the expectation is the first proposal will have wide support.
Six senators were holding calls Sunday afternoon to try to reach an accord on the issue after struggling for weeks. But ultimately, it will be up to the leadership to decide what to include in the spending bill that has to pass by Friday to keep the government open.
- 12/10/2020 – I think it is quite possible
Why the U.S. Economy Will Take Off in 2021
Three shocks battered the economy in 2020. Next year could be the start of a more resilient and sustainable boom.
The story of the U.S. economy in 2020 will consist of three major shocks: Covid, racial unrest and an election that divided the nation.
The story of 2021, however, will be of a great comeback.
But let’s start at the beginning, before the pandemic, when things seemed poised to go quite differently.
Unemployment was at record lows, and yet employment kept rising. The longest economic expansion in U.S. history appeared to have set off a virtuous cycle: Job gains led to increased household wealth and spending, which in turn encouraged more hiring, in some cases pulling off the sidelines people who had stopped looking for work.
Even more striking was that after years of widening, the wealth gap was shrinking. According to the Federal Reserve’s Survey of Consumer Finances, people in the lowest income quintile saw their net worth rise 37% from 2016 to 2019, while the top quintile largely held steady. Blacks and Hispanics, meanwhile, saw gains in net worth of 33% and 65%, respectively, while whites saw a gain of 3%.
- 12/20/2020 – still no deal. Let us wait for one more week.
POLITICS: McConnell rejects bipartisan Covid relief plan while House adjourns until next week
- Congress appears to be making little concrete progress toward agreeing to a coronavirus relief agreement.
- Lawmakers hope to approve more aid before lifelines expire at the end of the month, but issues including state and local aid, liability protections, unemployment assistance and stimulus checks are still dividing Congress.
- Lawmakers plan to pass a one-week government funding extension through Dec. 18 in order to buy more time to craft a pandemic rescue package and spending plan.
- 12/08/2020 – the negotiation on stimulus deal is dragging on
Lawmakers face hurdles to COVID relief deal
Negotiators in the House and Senate are racing to finish a massive end-of-year deal to fund the government and provide help to workers and families struggling through a worsening pandemic.
Last-minute sticking points are threatening to push the talks into the weekend or next week and may scuttle an agreement all together despite momentum for a deal that has been building since last week.
Congress is expected to pass a one-week stopgap measure as soon as Wednesday to keep the government funded through Dec. 18. Without such action, the government could shut down on Saturday.
Bipartisan COVID-19 relief deal faces congressional hurdles as McConnell refuses to endorse it
The $908B deal could fall apart unless GOP, Dems can compromise on key sticking point
Despite renewed momentum on Capitol Hill to pass a coronavirus relief deal, a bipartisan proposal unveiled last week has run into familiar obstacles, potentially thwarting the chances of a year-end agreement.
The biggest sticking points are ones that have plagued negotiations for months: Republicans maintain that a liability shield for businesses is needed, a “poison pill” for Democrats. At the same time, Democrats want to include billions in new funding for state and local governments, which their GOP colleagues have lambasted a “blue-state bailout.”
Unless the two sides can compromise on the issues, the $908 billion deal could fall apart.
ACG Analytics @ACGAnalytics We expect #Congress to pass a 1-week CR to extend government funding & allow more time for pandemic relief negotiations. We are increasingly optimistic that there will be a stimulus deal before Congress adjourns. #coronavirus
- 12/07/2020 – A bipartisan group of lawmakers hopes to release a more detailed outline of its $908 billion aid proposal on Monday as it prepares legislative text.
Congress looks for last-minute Covid stimulus deal as benefits cliff looms
- Lawmakers are rushing to craft both a coronavirus relief package and a government spending plan before the end of the week.
- Democratic leaders have backed a bipartisan $908 billion proposal as the basis for an emergency aid bill.
- An expansion of unemployment benefits and a moratorium on evictions will expire at the end of the month.
- 12/06/2020 – the state of recovery; good comprsion of stimulus bill, which one will pass?
Weighing the Week Ahead: What is the State of the Recovery?
Some indicators have shown significant improvement, as I regularly show in my reports on the Big Four in the Quant Corner. These are consumer spending and personal income. The former has changed in terms of venue and type of purchase but returned to pre-Covid levels. The latter has been bolstered by aid programs as well as the shift to work-from-home.
Many indicators – more than most realize – show a month-to-month comparison of how many respondents are doing better. There is no indication of how much the improvement was or how it compares to March. The most recent ISM reports, for example, provide absolutely no information on business conditions now as compared to March. That question is not even asked.
Important measures that have less direct stimulus – employment for example – have made a modest rebound from a deep drop. Taking the example above from Robert Dieli, it would take 40 months to fill the jobs gap at the current pace. And that pace is what we were seeing before the recession.
Recovery Timing and Vaccines
No one knows the exact answer to these questions, but the best estimates are six to eight months minimum and perhaps longer. Mr. Market thinks it will happen tomorrow!
Stimulus:
It is far to soon to declare victory on the passage of a stimulus bill, but there are finally some signs of progress. There is now a bipartisan coalition reaching some agreements. There is also pressure to act before the unemployment aid packages expire the day before Christmas.
Adding to the tension is the need for a budget resolution before the next government shutdown deadline, December 11th.
The partisan dispute is not just about the size of the package, although that is a factor. This helpful table shows the differing components between the bipartisan initiative and Sen. Majority Leader McConnell’s plan.

- 12/06/2020 – Trump and McConnell are ready to make the deal for stimulus plan, might be early this week
GOP’s Cassidy: Trump, McConnell Are ‘On Board’ With $908B Stimulus Relief Plan
President Donald Trump and Senate Majority Leader Mitch McConnell are “on board” with a $908 billion package to provide pandemic relief, according to a member of a bipartisan group that’s seeking legislation before the end of the year.
“President Trump has indicated that he would sign a $908 billion package — there’s only one $908 billion package out there and it’s ours,” Senator Bill Cassidy, a Republican from Louisiana, said on “Fox News Sunday.” “The pain of the American people is driving this and I’m optimistic that both of those leaders will come on board.”House Speaker Nancy Pelosi and Senate Democratic leader Chuck Schumer have endorsed using the bipartisan proposal as the basis for negotiations.
A final version of the proposed legislation could come early this week, Cassidy said.
A key sticking point is whether to provide liability protection to businesses whose workers fall ill to the coronavirus. Critics have said some companies, like meatpacking plants, shouldn’t be protected if there are indications they didn’t take adequate precautions. Cassidy said small companies could be driven out of business just from the cost of the gathering and exchanging evidence.
- 12/04/2020 – Stimulus plan gains more traction. That plan hasn’t yet been turned into legislative text — that won’t be finished until next week — and an agreement will hinge on details that have hung up a deal in the past. Pelosi, McConnell, Trump, Biden might be all on board.
GOP Lawmakers Warm to Bipartisan Stimulus Plan
Prospects for a pandemic relief package before the end of the year grew substantially as senior Republicans warmed to the idea of using a $908 billion proposal from a bipartisan group of lawmakers as a basis for a deal.
The plan outlined by Republican and Democrat lawmakers in the House and Senate has emerged as the first real chance for a compromise that has eluded party leaders and the White House for months.
Still, Senate Majority Leader Mitch McConnell hasn’t publicly thrown his support behind the plan, after having won President Donald Trump’s backing for his own, narrower proposal. That stance risks leaving him increasingly isolated as support shifts among Republicans eager to get some kind of agreement.
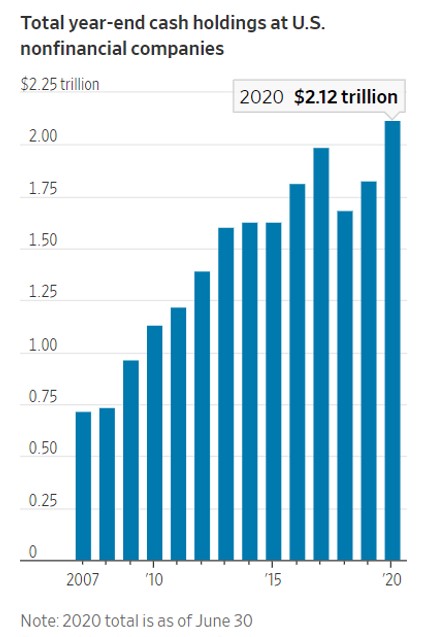
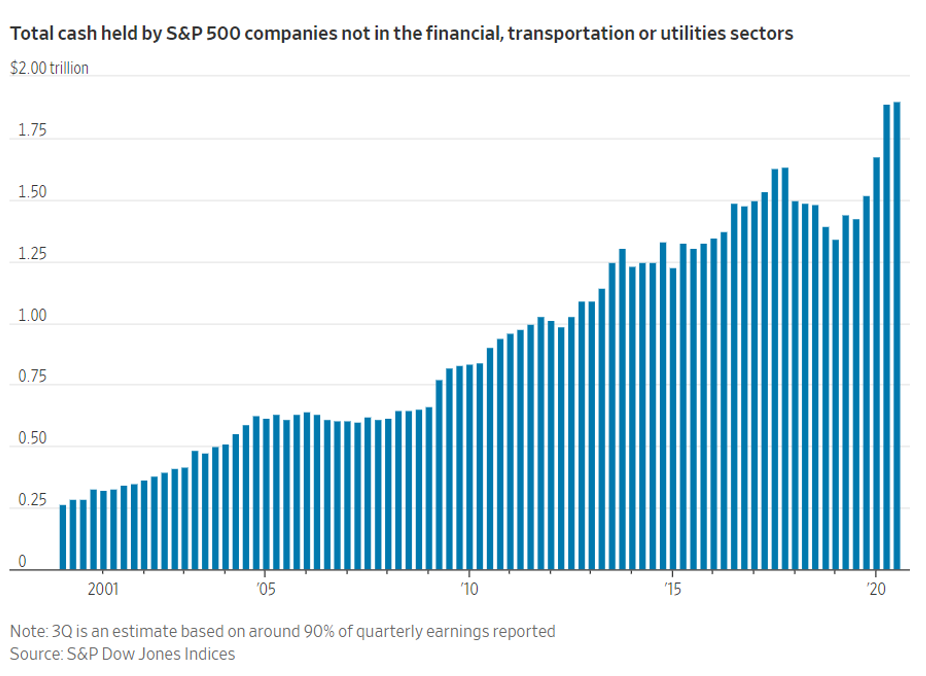
- 12/04/2020 – largest ever cash might induce lots of buybacks, investments, M&A in 2021. Watch out the opportunities going forward
Investors Circle Largest Corporate Cash Hoard Ever
Alternatives for companies include buybacks, capital projects, employee hiring, reducing debt and M&A
U.S. companies are sitting on the largest pile of cash ever. Investors are trying to gauge how they are going to use it.
Cash holdings at nonfinancial companies grew to a record $2.1 trillion at the end of June, according to a recent report from Moody’s Investors Service. That is up 30% from that time last year and higher than the previous peak of nearly $2 trillion in 2017. Among the biggest hoarders: AT&T Inc. T +1.56% and Delta Air Lines Inc., DAL -1.29% which each held more than $15 billion at the end of June.
Other measures show some of America’s largest companies continued to hang on to record cash stockpiles at the end of the third quarter. The amount held by S&P 500 companies not in the financial, transportation or utilities sectors is expected to total around $1.9 trillion, according to data compiled by S&P Dow Jones Indices. That is the most cash ever held by that group in data going back to 1980.
Cash hoards swelled this year after companies issued record-breaking amounts of debt to bolster their balance sheets against the Covid-19 pandemic’s disruptions. As of Nov. 30, companies had sold more than $2 trillion of investment-grade and high-yield bonds—the most on record in data going back to 2006—according to LCD, a unit of S&P Global Market Intelligence.
At the same time, many cut share repurchases, dividends or capital expenditures. Now that is starting to reverse, raising hopes for moves such as buybacks, which can drive share prices higher, and paying down debt, which reduces risk for bondholders.
Wall Street analysts expect companies to start dipping into more of their cash next year. Some investment-grade companies have taken initial steps to lower their debt loads, while continuing to hoard cash in anticipation of a surge in infections this winter, according to a BofA Securities report.
Some investors believe that firms will spend on capital projects or hire more employees rather than paying down debt, given that the Federal Reserve expects to keep interest rates near zero for the near future.
- 12/03/2020 – good sign for stimulus plan. Election is done, Pelosi sees no worry on giving credit to Trump before election for stimulus plan, in addition, the runoff in Georgia gives her more motivation to sign the deal. McConnell has no incentive to stall the deal. Trump too.
Pelosi and McConnell resume talks as Congress rushes to strike a Covid stimulus deal
- House Speaker Nancy Pelosi and Senate Majority Leader Mitch McConnell spoke Thursday as Congress rushes to strike a coronavirus stimulus deal and avoid a government shutdown.
- The pair talked “about their shared commitment to completing an omnibus [spending bill] and COVID relief as soon as possible,” Pelosi spokesman Drew Hammill said.
- McConnell earlier said he sees “hopeful signs” for reaching a relief agreement before the end of the year.
- Pelosi and Senate Minority Leader Chuck Schumer backed a bipartisan $908 billion stimulus package, while McConnell released his own roughly $500 billion plan.
- 12/02/2020 – negotiation on new stimulus plan is on going, Democrats (Pelosi etc) are willing to compromise, promising. Watch out relevant news before Dec. 11
Pelosi and Schumer back $900 billion coronavirus stimulus plan as basis for negotiations
- House Speaker Nancy Pelosi and Senate Minority Leader Chuck Schumer urged Senate Majority Leader Mitch McConnell to use a $908 billion bipartisan stimulus plan as the basis for relief talks, endorsing a smaller relief bill than they previously have.
- McConnell and House Majority Leader Steny Hoyer were set to discuss coronavirus stimulus Wednesday for the second time this week.
- Lawmakers are rushing to pass a pandemic relief package before the end of the year as the outbreak rages around the country.
- McConnell and Hoyer both signaled they could include aid provisions in a spending bill Congress needs to pass by Dec. 11.
Coronavirus Stimulus Talks Moving in Right Direction, Party Leaders Say
State and local aid as well as liability protections remain hurdles in negotiations
“The number is not the problem,” said Sen. Lindsey Graham (R., S.C.). “It’s policy differences.”
Mr. Graham, a new voice in support of the bipartisan proposal, said he had spoken about the package with President Trump. “The president’s of the mind-set a bill would be good for the country, he would like to see it happen, but it’s got to have the right policy,” he said.
Both Mr. Trump and President-elect Joe Biden have urged Congress to reach a deal. “I think we are getting very close,” Mr. Trump said Thursday. “I want it to happen. And I believe we are getting very close to a deal.”
- 12/02/2020 – is the new stimulus plan coming before the end of next week?
Coronavirus Stimulus Efforts Show Momentum
Republicans and Democrats signal desire to reach deal soon
WASHINGTON—The partisan standoff over a new coronavirus-aid package showed new hints of thawing Wednesday, as Republicans said they saw signs that Democrats were seeking a compromise.
Senate Majority Leader Mitch McConnell (R., Ky.) said Wednesday that Democratic leaders had “signaled a new willingness to engage in good faith.” On Monday night, House Speaker Nancy Pelosi (D., Calif.) and Senate Minority Leader Chuck Schumer (D., N.Y.) sent Mr. McConnell a new Covid-19 relief proposal, whose contents they have declined to disclose.
House Majority Leader Steny Hoyer (D., Md.) said Wednesday that he was hopeful that in the next few days, “we will be able to come to an agreement on a bill that responds to these major crises, at least in the short term,” referencing the need to help state and local municipalities and small businesses, among other things.
Mr. Hoyer said he had spoken to Mr. McConnell and the two agreed that legislation should come up before the end of next week.
- 11/25/2020 – Davidson thinks we are in in early stages of the shift favoring what can be called the US core economy issues. So after vaccine is clear, we might just start the reset. I might still have some opportunities to invest.
In my opinion, we are in early stages of the shift favoring what can be called the US core economy issues. That oil prices are rapidly approaching $45/BBL is a reflection of this shift in market psychology.
- 11/18/2020 – application for EUA might be this Friday
BioNTech and Pfizer will seek emergency US authorization for vaccine on Friday, CEO says
- 11/18/2020 – it seems like EUA is coming next week
Pfizer Says Vaccine Is 95% Effective in Final Data, Will Seek Authorization
Shot could be on track to go into distribution by the end of the year if health regulators permit
Out of 170 adult volunteers in the nearly 44,000-subject trial who developed Covid-19 with at least one symptom, 162 received a placebo, while eight got the vaccine, Pfizer and BioNTech said.
The resulting 95% effectiveness rate puts the shot’s performance on par with shingles and measles vaccines. It is also consistent with the vaccine’s showing in a peek last week at how it did in an analysis of the first 94 subjects to fall sick.
Researchers haven’t found any serious safety issues, the companies said. The vaccine appeared to be well tolerated following a review of data from 8,000 study subjects, the companies said.
A severe side effect was fatigue, reported by 3.8% of the subjects, the companies said. Also, 2% of subjects reported headaches.
The companies said they have collected the two months of safety data on about 19,000 study subjects requested by the U.S. Food and Drug Administration but are still reviewing all those results.
Days earlier, Moderna Inc. reported similarly strong preliminary results for its shot, which the biotech said was 94.5% effective in an early look.
The vaccine was effective across different ages, races and ethnic groups, and it was more than 94% effective in adults over 65 years old, the companies said.
About 42% of the trial participants are from racial or ethnic minority groups, while 41% are ages 56 to 85, the companies said.
- 11/17/2020 – watch out the upcoming (days away, the third week of November) EUA application and approval, and EUA might be approved in the first half of December. This could be short term catalyst for beaten down stocks like CCL, AMC or PFE, MRNA. I might can play with LEAP
According to cnn news, Trump admin plans to roll out vaccine from Pfizer and Moderna at the same time!
Pfizer ‘Very Close’ to Applying for US Emergency Approval, Says CEO
Read Newsmax: Pfizer ‘very Close’ to Applying for US Emergency Approval, Says CEO | Newsmax.com
Pfizer is “very close” to applying for an emergency use approval for its Covid-19 vaccine after collecting safety data to submit to US regulators, the company’s CEO said Tuesday, according to a report.
The pharmaceutical giant announced last week preliminary results from a late-stage clinical trial showing the injections it had co-developed with Germany’s BioNTech was more than 90 percent effective after the second dose.
“We are very close to submitting for an emergency use authorization,” Albert Bourla told medical news site Stat. “We will announce it as soon as we are doing it.”
Pfizer (PFE) has previously said it expects to contact the US Food and Drug Administration to apply for an Emergency Use Authorization by the third week of November, meaning the announcement could be days away.
Moderna has previously said it expects to apply for an EUA by November 25.
After the companies apply, the EUAs could follow in a matter of weeks.
Moncef Slaoui, chief of the US government’s Operation Warp Speed for vaccine and treatment development, said he expects approval in the first half of December.
Since companies that have been funded by the government have already been manufacturing their doses ahead of approval, Slaoui says there will be enough between Pfizer and Moderna to immunize 20 million Americans in December.
The FDA has scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee, a group of outside experts, for December 8, 9 and 10, a source familiar with the process told CNN Tuesday.
The agency could make a decision at the end of the meeting on December 10 about whether to issue emergency use authorizations for the vaccines, the source said.
“It will make sense that in all likelihood the FDA will consider both applications together,” the source said, considering that both vaccines use the same technology and appear to have very similar safety and efficacy results from their large-scale Phase 3 clinical trials.
- 11/16/2020 – Great news from Moderna. I read about this news (from Fauci’s comments, etc) a few days ago, I should have bought in-the-money short term LEAP (one month) for it, then I would have 10X return today! Big mistake I missed this action.
Moderna CEO cheers coronavirus vaccine safety as ‘gamechanger,’ with 20M doses available by end of year
‘People who did got our vaccine did not get any severe disease’
Bancel described the vaccine as “user-friendly” because it can last six months in a regular freezer and seven days in a refrigerator. The Pfizer-BioNTech coronavirus vaccine, which was found to be more than 90% effective in preventing coronavirus in the companies’ Phase 3 clinical trial, must be kept in significantly colder temperatures, making it harder to distribute.
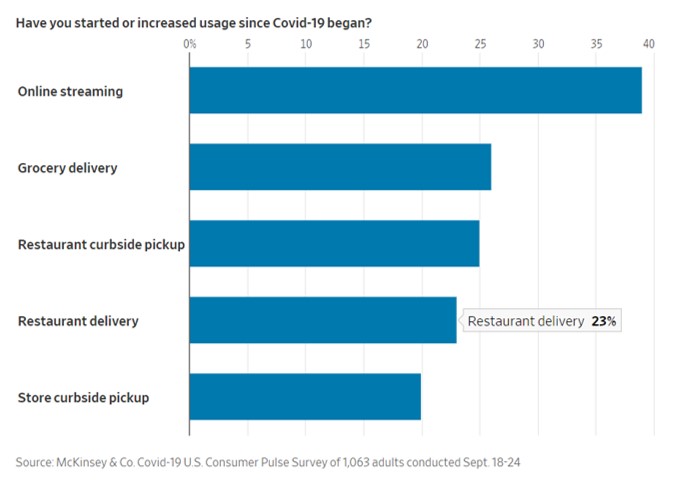
- 11/15/2020 – the change from physical to virtual looks more lasting and spans generations. It seems like e-commerce will have more potential for growth.
Pandemic Speeds Americans’ Embrace of Digital Commerce
Consumers try new ways to get health care, buy vehicles, eat and work out as the pandemic shakes up habits
The pandemic’s disruptions have transformed how American consumers behave by accelerating their embrace of digital commerce, and the changes are likely to prove permanent, according to businesses studying and adapting to the changes.
A recent survey by consulting firm McKinsey & Co. found that about three out of four people have tried a new shopping method due to the coronavirus and that more than half of all consumers intend to continue using curbside pickup and grocery-delivery services after the pandemic is over. Nearly 70% of consumers surveyed intend to continue buying online for store pickup.
The pandemic collapsed into three months a process of adopting e-commerce that otherwise would have taken 10 years in the U.S., the firm concluded.
the change in how they buy things looks more lasting and spans generations.
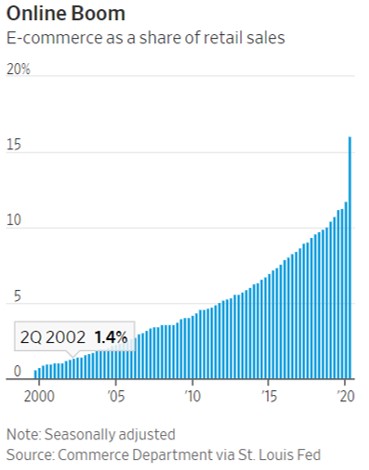
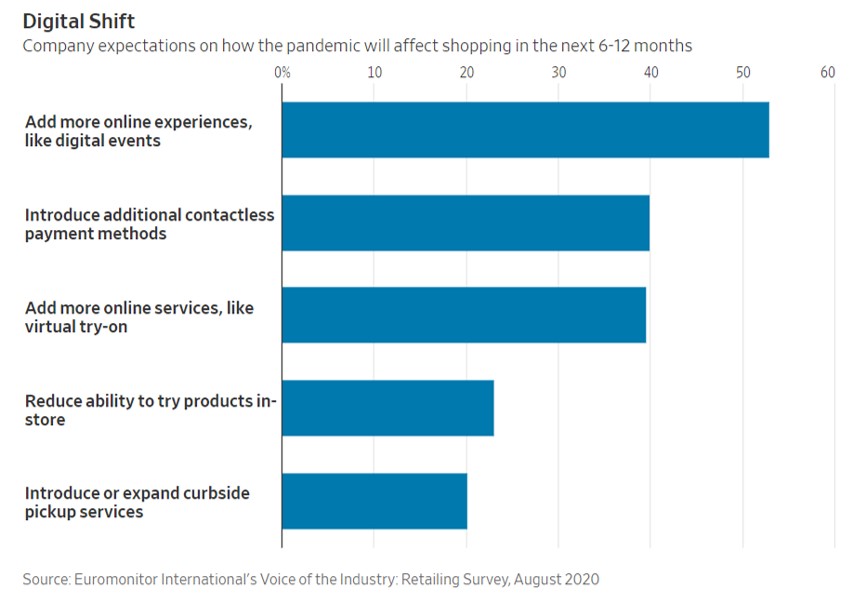
- 11/14/2020 – interesting to know that drastically change of stock on vaccine hope on Monday Nov. 09. Some of the change were reversed on Tuesday, I might can still find some opportunities.
Weighing the Week Ahead: Learning from Swiss Cheese
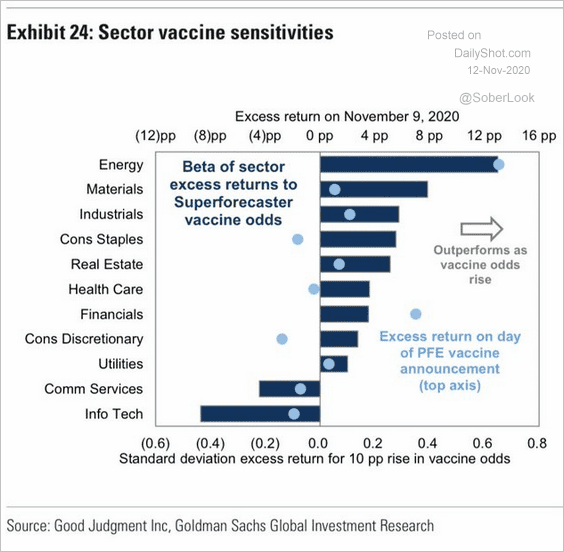
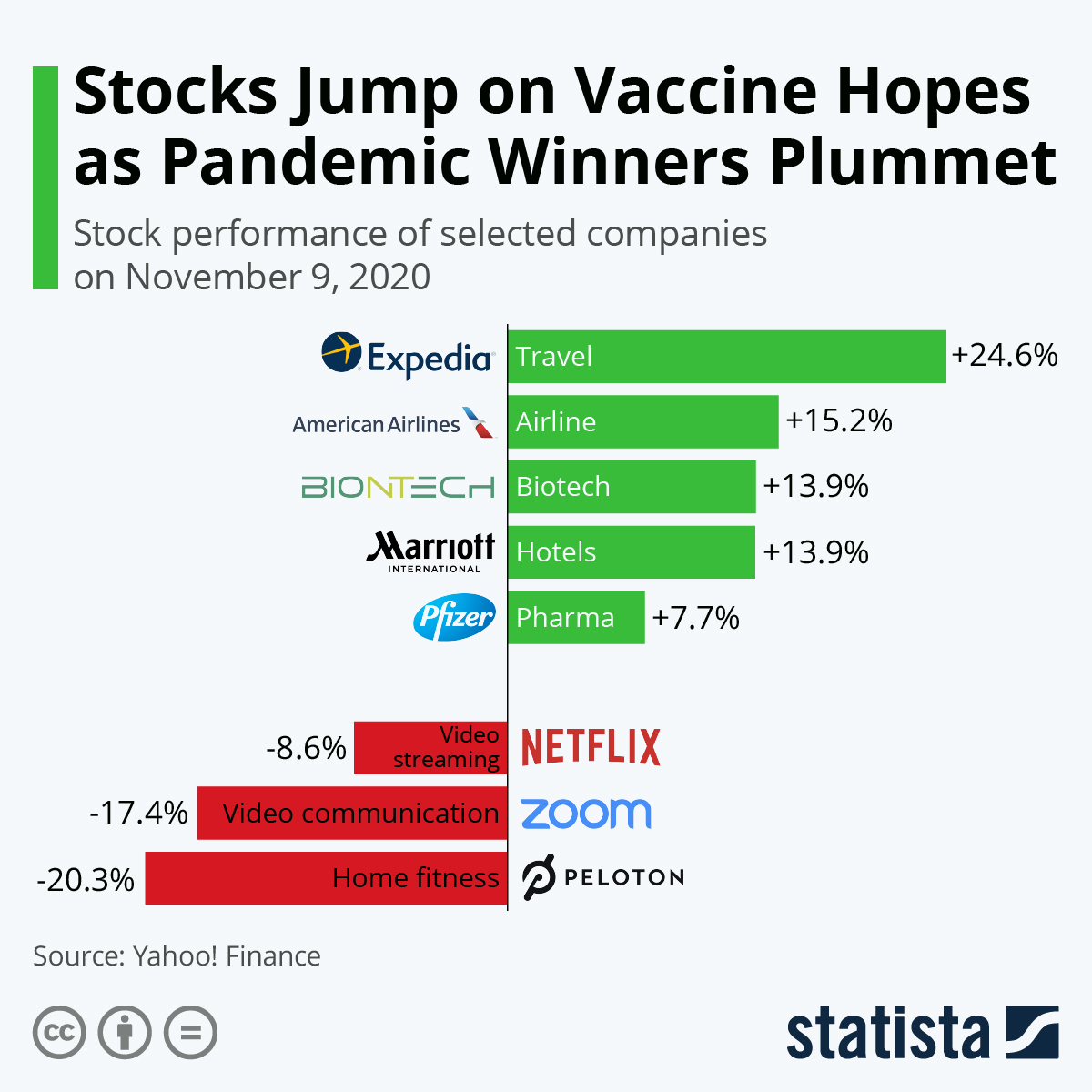
This did not capture the cruise line moves – up 30%!
Mr. Market changed his mind on Tuesday, and some of the effect was reversed. Cruise lines were down 10%. It was exciting but not predictable.
- 11/12/2020 -BioNTech/Pfizer vaccine might can stop the CV-19 and might can be distributed to the whole world very fast due to the significant demand
really encourage news from BioNTech.

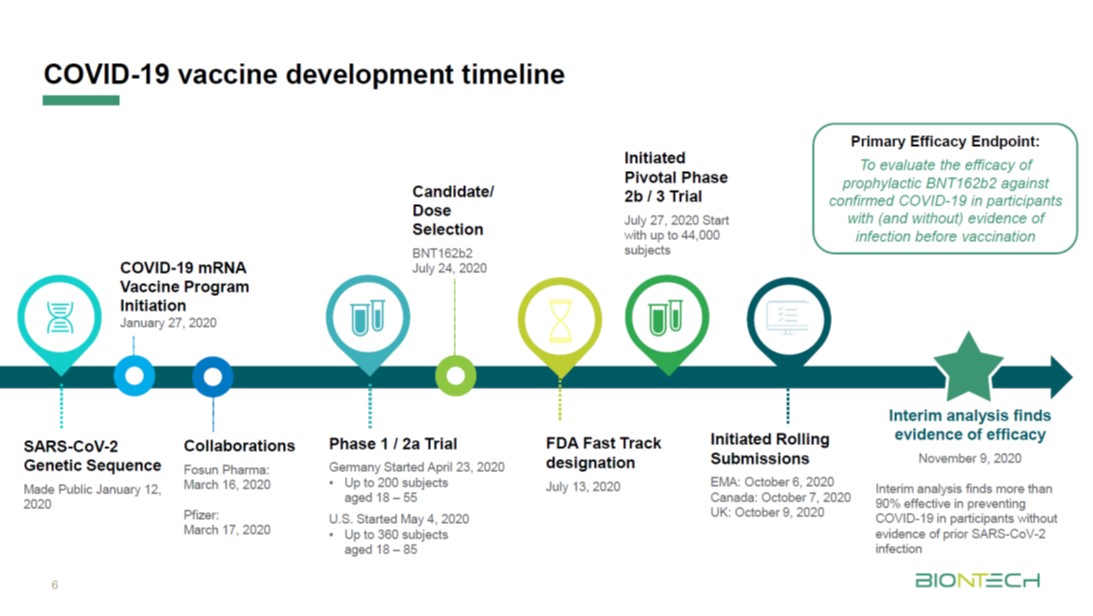
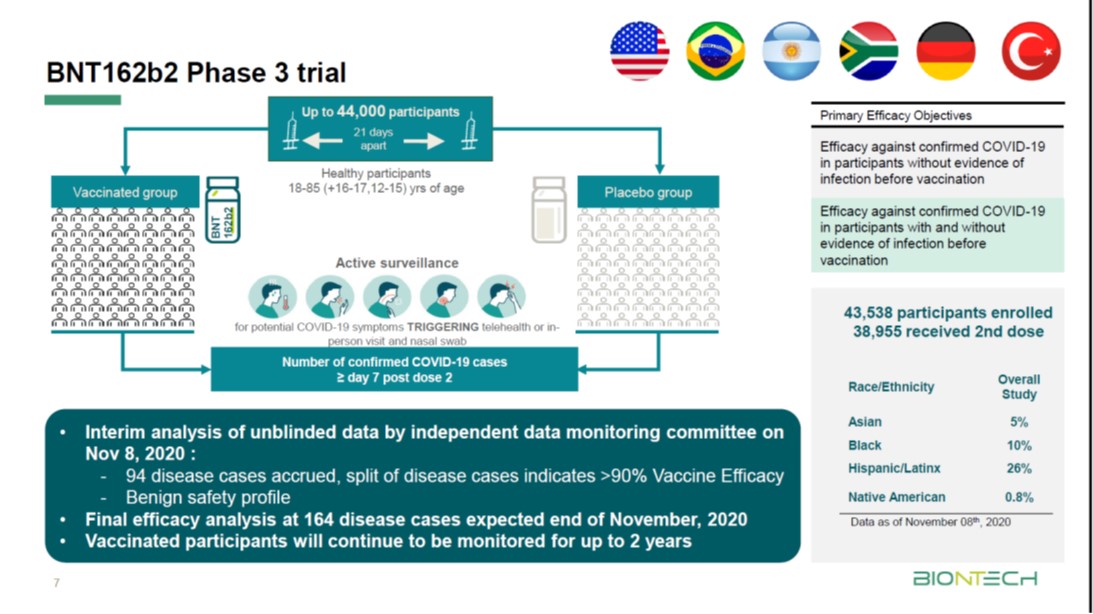
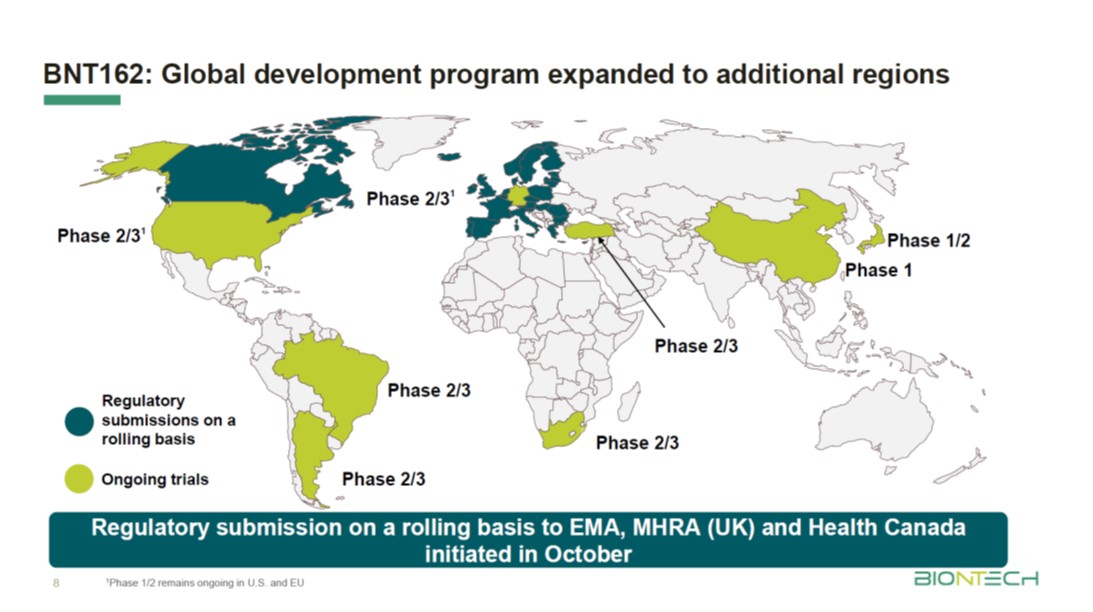
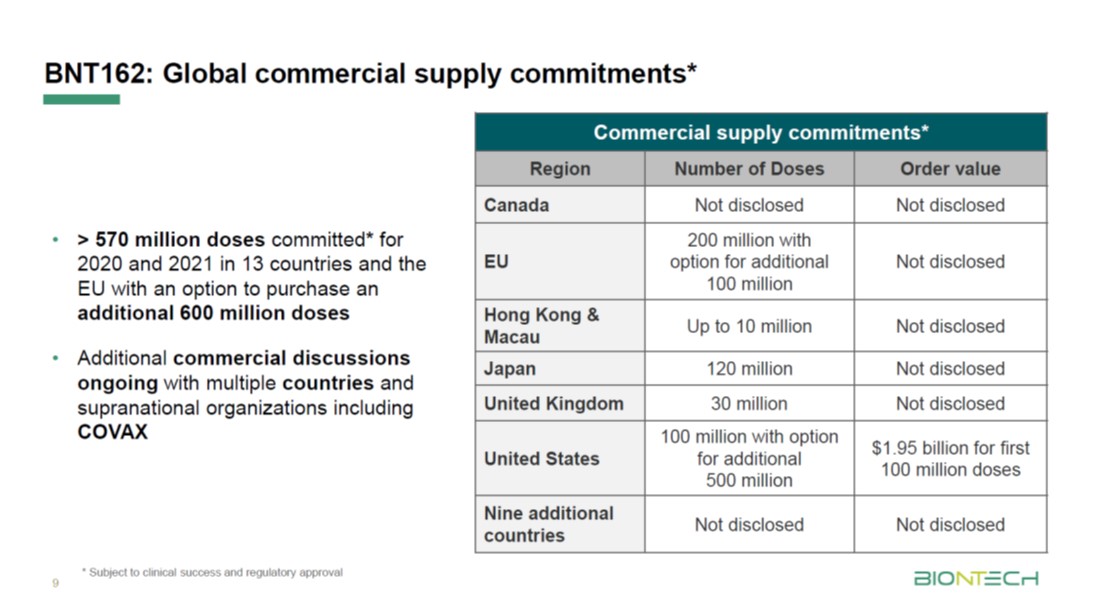
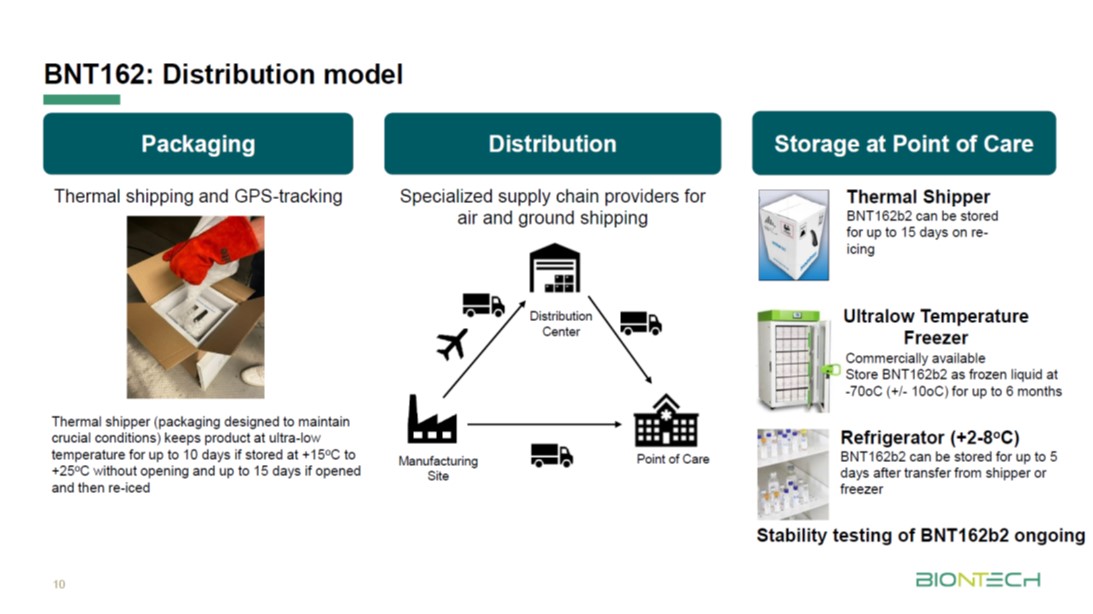
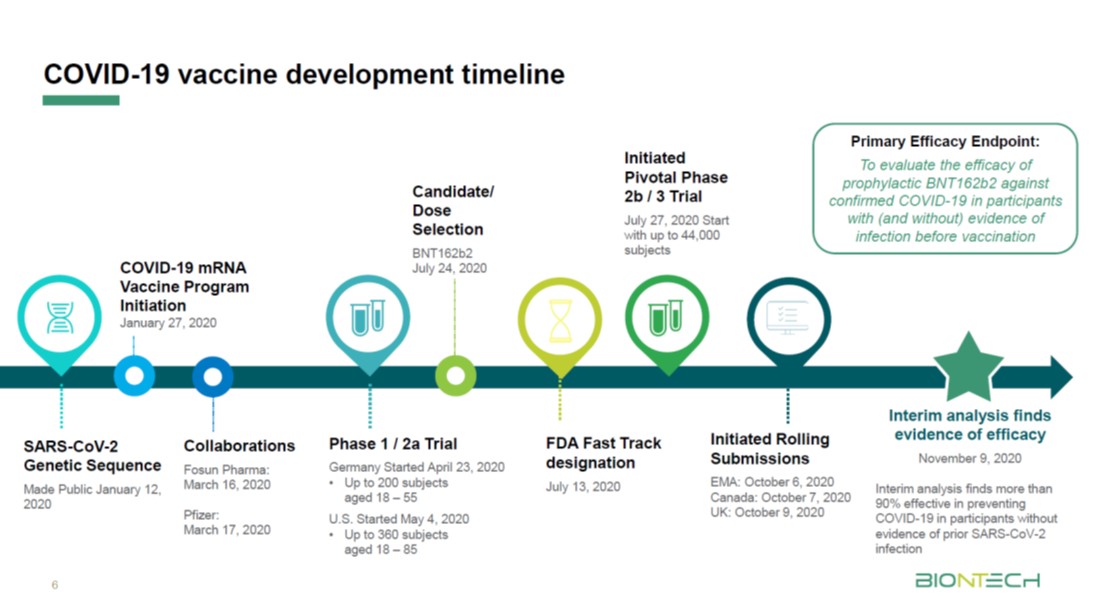
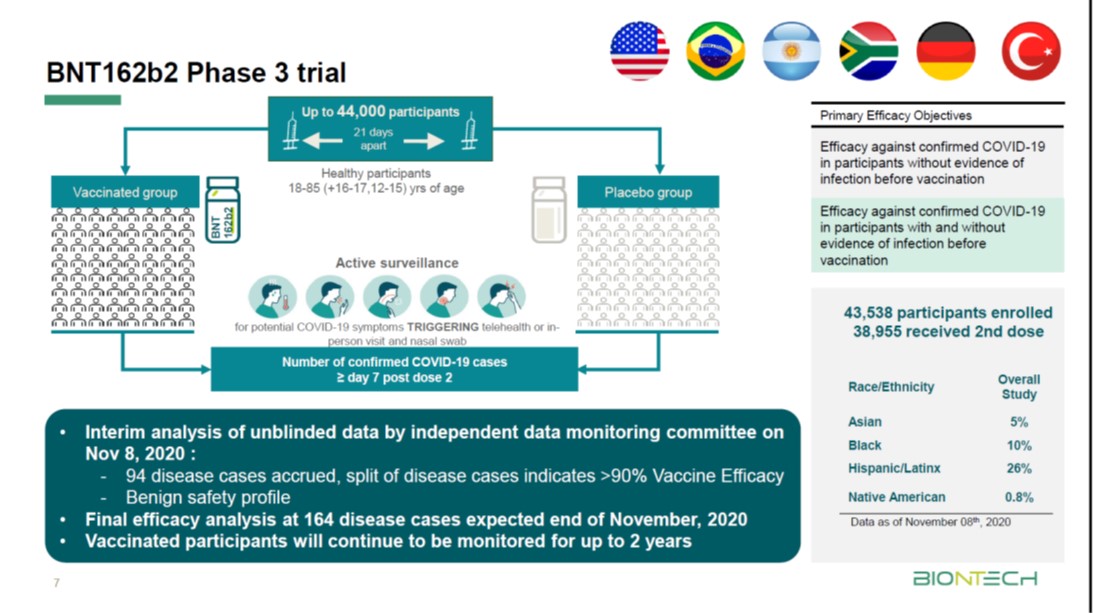
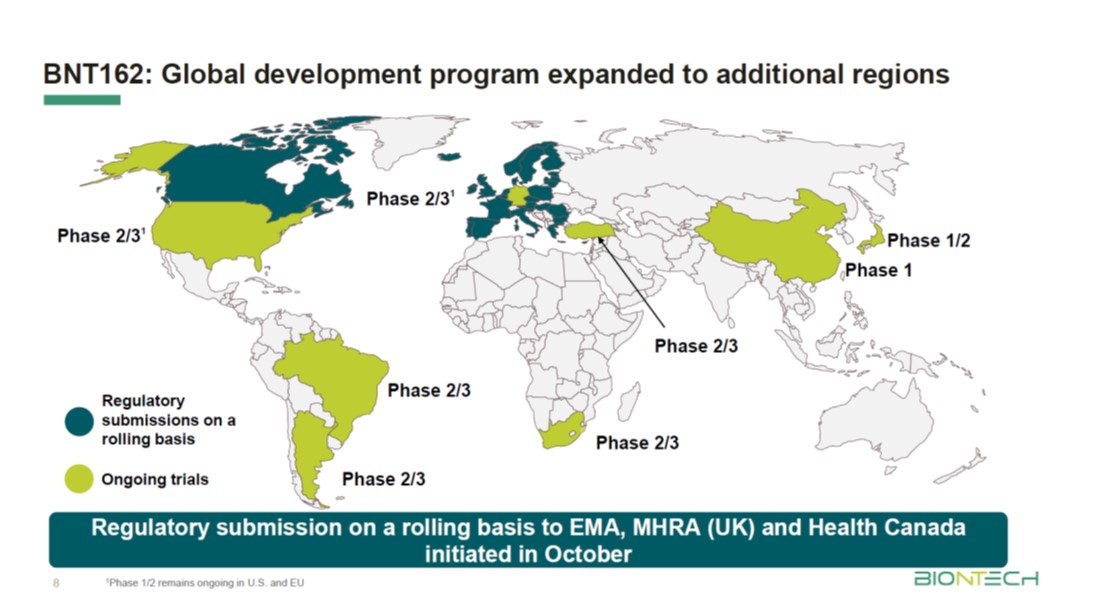
Pfizer coronavirus vaccine expected to protect patients for a year: BioNTech founder
Pfizer and BioNTech’s vaccine was found to be 90% effective
The Pfizer-BioNTech coronavirus vaccine could protect patients from the virus for a year, BioNTech CEO Ugur Sahin told “Mornings with Maria” on Thursday.
“There’s a good chance that the vaccine not only is protective but could protect from infection or reinfection for a significant period of time,” Sahin said. “I personally expect that a vaccine could protect us … for at least one year. If we learn that we need to reimmunize, we could do that after one year.”
Pfizer and BioNTech’s vaccine was found to be more than 90% effective in preventing coronavirus in their Phase 3 clinical trial.
“This is indeed unprecedented,” Sahin said. “We started vaccine candidates development end of January, about 10 months ago in Germany, by starting to evaluate more than 20 candidates. In March, we announced our partnership with Pfizer.”
“The speed was only possible by really expanded cooperation and by using the lag times or essentially the principle that we don’t have time to lose,” he continued. “We did not cut corners. We went through several clinical trials. We did clinical trials in Germany. We did clinical trials in the United States. It was executed by our colleagues from Pfizer, with more than 43,000 subjects so far.”
The development is a significant step toward combating the global pandemic at a time when many areas are seeing a rise in cases.
Scientist behind BioNTech/Pfizer vaccine says it can end pandemic
The scientist behind the first Covid-19 vaccine to clear interim clinical trials says he is confident his product can “bash the virus over the head” and put an end to the pandemic that has held the world hostage in 2020.
The most effective candidate to emerge from the company’s trials, Şahin said, attacked the coronavirus “in more ways than one”.
“The vaccine hinders Covid-19 from gaining access to our cells. But even if the virus manages to find a way in, then the T-cells bash it over the head and eliminate it. We have trained the immune system very well to perfect these two defensive moves. We now know that the virus can’t defend itself against these mechanisms.”
Some crucial questions regarding the vaccine’s efficacy will only be answerable in the coming weeks and months, Şahin said. Establishing for certain whether it can also stop asymptomatic infections could take up to a year.
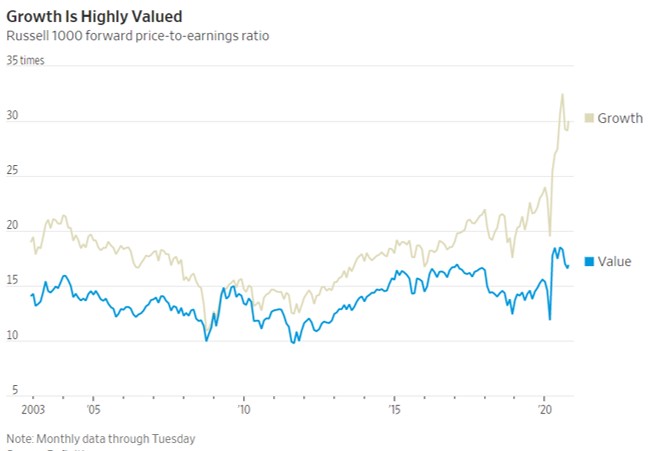
- 11/12/2020 – value stocks lags behind growth since 2006
Value No Longer ValuedCheap or ‘value’ stocks beat growth stocks hands-down for decades—until 2006
Pfizer’s Vaccine Is a Pick-Me-Up for Value Stocks
For sustained outperformance, value stocks need to have been depressed beyond what is justified by their poor fundamentals
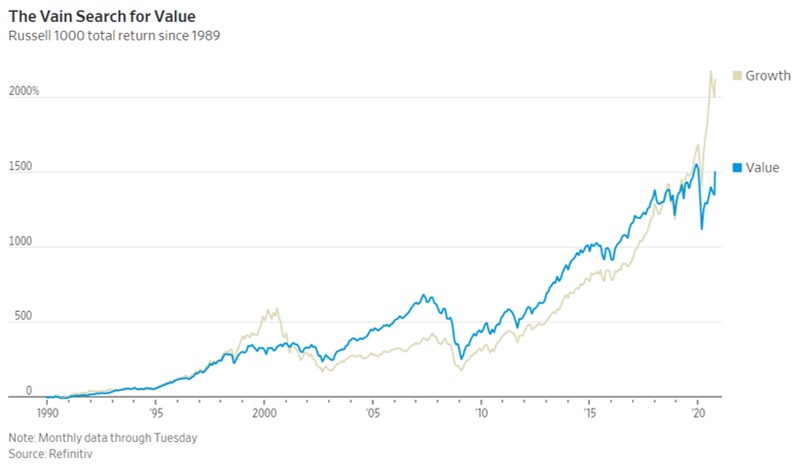
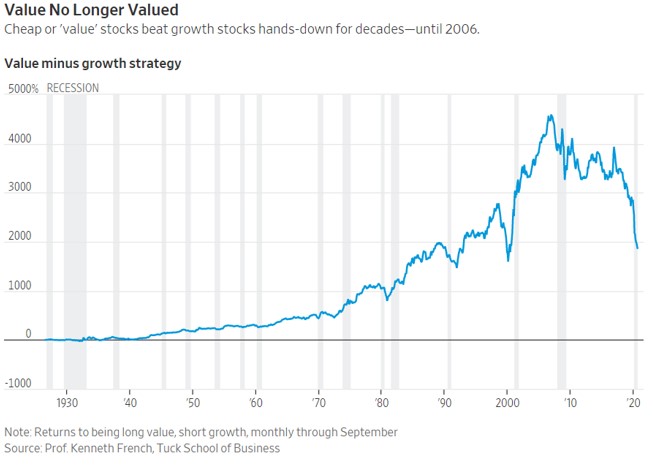
History also shows that value’s big rebounds have often proved temporary. The six times in the 1930s when value beat growth by even more than on Monday mostly ran for a few months, then fizzled; only value’s big win in the buildup to the election of Franklin D. Roosevelt in 1932 lasted more than a year, and that was volatile. Value has had several false dawns in the past decade too, most clearly for a year after the European Central Bank promised to save the euro in July 2012, and for a couple of months after Donald Trump’s win in 2016.
After the 2016 election, “value stocks took off and left us for dust,” said Cathie Wood, chief executive of growth investment firm ARK Investment Management. “So we’ve been here before, and it was just the launchpad for 2017, which was one of the most spectacular years for us.”
- 11/12/2020 – review of market crashes: the 2020 market’s recovery took only 126 days – by far the fastest on record
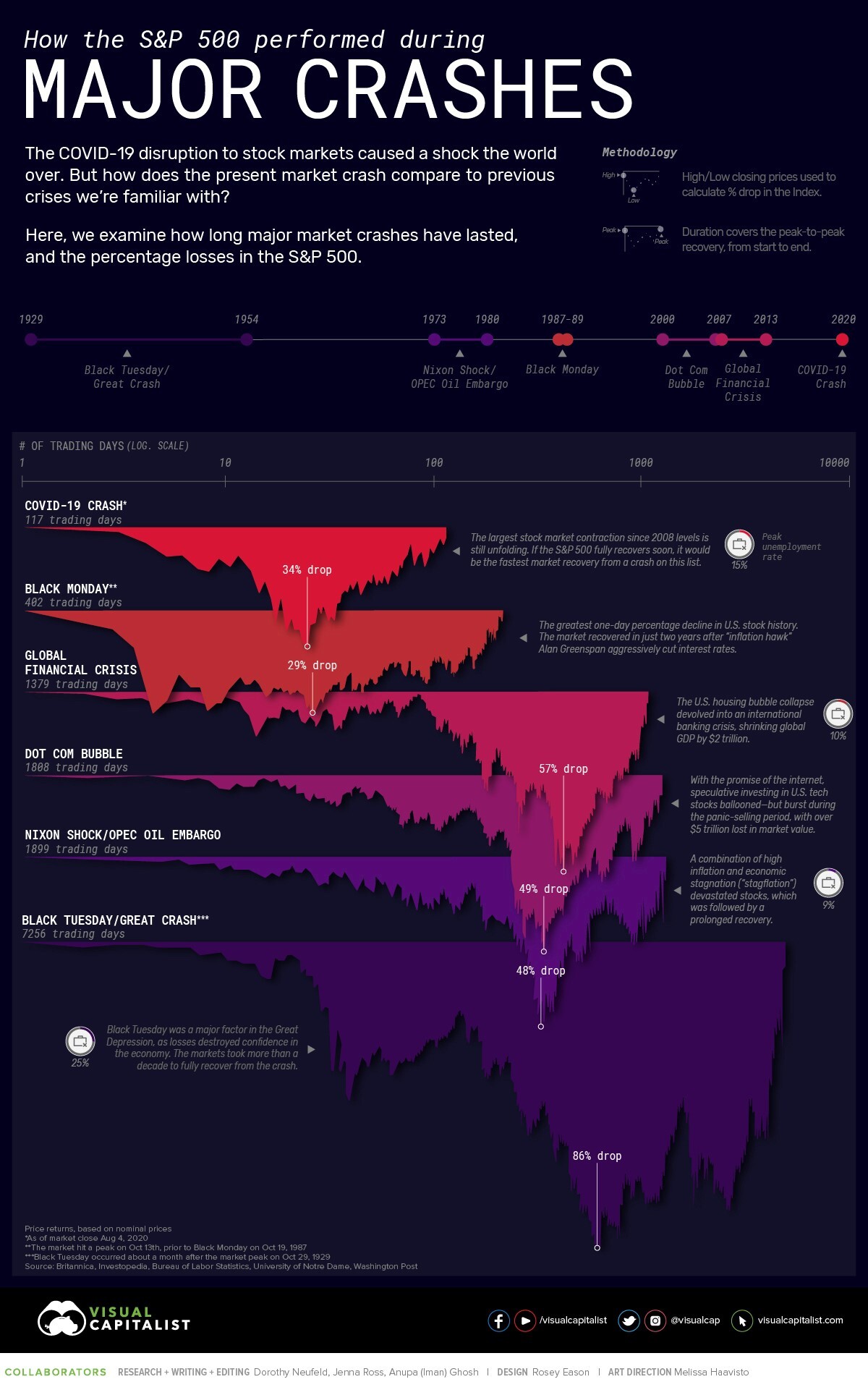
Though it’s three months out of date, this is nevertheless an interesting chart from VisualCapitalist showing how rapid the COVID-19 crash and recovery was relative to five other major market crashes over the past century:
Note that the S&P 500 Index surpassed its prior all-time high set in February on August 18, only nine trading days after this chart was published. This means that the market’s recovery took only 126 days – by far the fastest on record.
- 11/11/2020 – still some unknowns and challenges on the vaccine, but Pfizer’s vaccine sets the right direction for the end of pandemic
Pfizer’s and BioNTech’s vaccine is the start of the end of the pandemic
Its 90% effectiveness is as good as it gets, and bodes well for other vaccines. But getting them quickly to the right people will be hard
The Pfizer-BioNTech vaccine relies on a technology known as messenger RNA, or mRNA. The jab injects genetic material from the virus into the body, which uses this material to create a protein normally seen on the surface of covid virus particles, which in turn stimulates the immune system. It is being tested on an ethnically diverse group of 43,000 people, and the trial is not yet complete. The results announced so far are based on an interim analysis conducted by an independent data-monitoring group. The firms plan to submit their data for review in a scientific publication. It is possible that the efficacy estimate could change, as further data are gathered. That said, the results are sufficiently remarkable that it is unlikely that the final outcome will be anything other than an extremely useful vaccine.
Three important questions about the vaccine remain. One is the extent to which it works in elderly people, one of the groups most vulnerable to covid-19, and who may not respond as well. Another is whether it prevents infectiousness (it remains possible that a vaccine could prevent someone from getting the symptoms of covid-19, but not from spreading it to others). And its long-term efficacy is entirely unknown.
Even so, there is little doubt that the findings are enormously positive. Moreover, Pfizer says that no serious safety concerns have arisen in the trials under way, although further efficacy data are being collected.
News about two more vaccines, from AstraZeneca, another big pharma company, with a team at Oxford University, and Moderna, an American biotechnology company, is also expected in the coming weeks. The AstraZeneca-Oxford vaccine is already known to stimulate a good immune response in the elderly. Even if Pfizer’s vaccine does not do so well in this group, therefore, there is a good chance that another will do this job.
In short, the arrival of vaccines to tame the pandemic is now within reach. But it will take time. The next step will be for Pfizer to apply for emergency authorisation for the vaccine in America and Europe. The World Health Organisation (WHO) has a process for allowing such authorisations to be used in countries without regulatory agencies. The application for BNT162b2 will have to wait until the third week of November. Pfizer will not apply until it has gathered two months of safety data from participants in the trial. Agencies might authorise it for use in high-risk groups (eg, hospital doctors and nurses) by the end of the year, pending further safety data; broader approval could come in the first quarter of 2021. Supplies of vaccines will also be limited at first, even though mass manufacturing of BNT162b2 has been under way since October. Current projections suggest 50m vaccine doses will be available in 2020, and 1.3bn in 2021.
And there is one other cause for celebrating. The mRNA approach that Pfizer and BioNTech are using has never been shown to work in humans before. The data gathered from the large-scale trials of this “platform” technology mean the firms can quickly and easily make minor revisions to the mRNA sequence, thus changing the proteins the body develops immunity to. This means that if new strains of covid-19 emerge, appropriate revisions of the vaccine could be created rapidly to contain it
- 11/11/2020 – should I also be quite cautious on this?
Ackman Places New Bet Against Corporate Credit
Hedge fund manager Bill Ackman has put on another bet that companies will struggle to pay their debts, just eight months after he cashed in a $2.6bn profit from a similar trade at the start of the pandemic.
The founder of Pershing Square told attendees at the Financial Times’ Dealmakers conference on Tuesday that markets had once again become too complacent about the coronavirus.
At the start of this week he put on a new trade hedging his equity exposure with insurance against corporate defaults, he said.
“I hope we lose money on this next hedge,” Mr Ackman said. “We’re in a treacherous time generally and what’s fascinating is the same bet we put on eight months ago is available on the same terms as if there had never been a fire and on the probability that the world is going to be fine.”
He said the new hedge is close to 30 per cent the size of the bet he placed in late February, when he bought a set of huge insurance policies linked to $71bn of corporate debt.
The billionaire investor anticipated that governments would be forced to shut down large swaths of their economies in order to curb the spread of coronavirus, leaving many indebted companies exposed. When that swiftly proved accurate, the value of the insurance ballooned and Pershing Square exited the trade in mid-March, pocketing $2.6bn in profits after having only paid $27m in premiums.
- 11/11/2020 – is it exaggeration or real? Is it the reason Ackman hedge his portfolio?
U.S. prepares for worst four months of the pandemic as it stares down the ‘darkest’ days yet
“Unfortunately, the worst days are ahead of us,” Mokdad said. “We are starting from a worse position, because we didn’t do a good job in the summer to bring it down and then we see right now a rapid rise in cases, so the surge of fall and winter has started. That’s why the worst days are ahead of us.”
To be sure, the U.S. has more tools to fight the virus than ever before. Pfizer and BioNTech released early data from their late-stage vaccine trial on Monday that indicated it was more than 90% effective. If authorized, the vaccine could be available to a limited number of people as early as December, said Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases. Pfizer said it can make enough doses of its two-dose vaccine to immunize about 25 million out of roughly 331 million Americans before the end of the year.
- 11/11/2020 – this could be very detrimental to the economy in the short run, also might not ensure virus will be gone in 4~6 weeks
Biden COVID-19 adviser suggests potential lockdown lasting over a month Doctor declares it would protect the economy until vaccines are ready for release
“When we were at 32,000 cases a day in the United States, people thought that maybe things were going to start going away,” he told Minnesota Public Radio Monday. “As you saw, we’re now at 125,000 cases per day. We’re seeing hospitals right now completely overwhelmed in a number of states.”
Those numbers will continue to climb, he predicted, for at least the next two to three months.
- 11/11/2020 -CEO’s cash out of 60% of stocks seems like not very confident at the final trial outcome. However, since the sale is “part of a predetermined trading plan adopted August 19”, maybe this is not a sign of weakness. On the other hand, I might need to learn from this CEO to be very discipline on my sale of stocks.
- Pfizer CEO Albert Bourla sold 62% of his stock in the company on the same day the drugmaker announced the results of its COVID-19 vaccine trial.
- Bourla sold $5.6 million in stock on Monday as part of a predetermined trading plan adopted August 19.
- His stock sale was carried out at $41.94 a share. The 52-week-high for Pfizer stock is $41.99, which means the CEO cashed out his shares at close to their highest price this year.
- Pfizer and its German partner BioNTech on Monday became the first to post positive results from late-stage COVID-19 vaccine trials.
- 11/11/2020 – be aware and be prepared
- The data released by Pfizer Monday was delivered in a news release, not a peer-reviewed medical journal. It is not conclusive evidence that the vaccine is safe and effective, and the initial finding of more than 90 percent efficacy could change as the trial goes on.
-
the news that Pfizer’s trial was progressing quickly was a good sign for other trials. “If there’s any silver lining in the fact that our country is currently on fire with this virus, it’s that these trials can reach a conclusion much quicker than otherwise,” he said.
Pfizer’s Early Data Shows Vaccine Is More Than 90% Effective
Pfizer announced positive early results from its coronavirus vaccine trial, cementing the lead in a frenzied global race that has unfolded at record-breaking speed.
Independent scientists have cautioned against hyping early results before long-term safety and efficacy data has been collected. And no one knows how long the vaccine’s protection might last. Still, the development makes Pfizer the first company to announce positive results from a late-stage vaccine trial, vaulting it to the front of a frenzied global race that began in January and has unfolded at record-breaking speed.
Dr. Paul Offit, a professor at the University of Pennsylvania and a member of the F.D.A.’s vaccine advisory panel, said the news that Pfizer’s trial was progressing quickly was a good sign for other trials.
“If there’s any silver lining in the fact that our country is currently on fire with this virus, it’s that these trials can reach a conclusion much quicker than otherwise,” he said.
Dr. Jansen said she learned of the results from the outside panel of experts shortly after 1 p.m. on Sunday, and that the timing was not influenced by the election. “We have always said that science is driving how we conduct ourselves — no politics,” she said.
The data released by Pfizer Monday was delivered in a news release, not a peer-reviewed medical journal. It is not conclusive evidence that the vaccine is safe and effective, and the initial finding of more than 90 percent efficacy could change as the trial goes on.
“We need to see the actual data, and we’re going to need longer-term results,” said Jesse Goodman, a professor of medicine and infectious diseases at Georgetown University. Still, he said, “it’s a testament to hard work and science that we’re getting results that are so good and so fast.”
Other scientists were stunned by the data so far.
“This is really a spectacular number,” said Akiko Iwasaki, an immunologist at Yale University. “I wasn’t expecting it to be this high. I was preparing myself for something like 55 percent.”
- 11/10/2020 – serious side effects of Pfizer’s vaccine, will this cause some market fear in the future so that I can find the opportunity to buy in more?
‘It’s like a severe hangover’: Volunteers who were first in the world to be given Pfizer’s Covid vaccine reveal how the side effects gave them headaches and left them ‘aching all over’ More than 43,500 people from six countries have taken part in the Pfizer trial
Carrier, 45, from the US revealed she felt it was her ‘civic duty’ to take part
And Glenn Deshields, a lobbyist from US, compared affects to ‘hangover’
- 11/09/2020 – CV-19 therapeutic medicine from JJ is approved too
FDA clears Eli Lilly COVID-19 antibody treatment for emergency use
The US government has purchased 300,000 doses for $375M
- 11/09/2020 – the vaccine might come. Pfizer said it remained on track to collect at least two months of safety data during the third week of November and could file for an emergency authorization shortly thereafter.
Pfizer’s Covid-19 Vaccine Proves 90% Effective in Latest Trials
Drugmaker and partner BioNTech could seek FDA authorization by end of November
A vaccine developed by Pfizer Inc. PFE 11.32% and partner BioNTech SE BNTX 12.85% proved better than expected at protecting people from Covid-19 in a pivotal study, a milestone in the hunt for shots that can stop the global pandemic.
The vaccine proved to be more than 90% effective in the first 94 subjects who were infected by the new coronavirus and developed at least one symptom, the companies said Monday.
The positive, though incomplete, results bring the vaccine a big step closer to getting cleared for widespread use.
Pfizer said it is on track to ask health regulators for permission to sell the shot before the end of this month, if pending data indicate the vaccine is safe.
The findings arrived on the timetable that the companies had been projecting. The results came too early for researchers to assess the safety of the vaccine, which the U.S. Food and Drug Administration says must include two months of monitoring at least half the study’s subjects for side effects.
Pfizer said it remained on track to collect at least two months of safety data during the third week of November and could file for an emergency authorization shortly thereafter.
Everything We Know About Pfizer’s Covid-19 Vaccine
- 10/27/2020 – BioNTech does not look like a fraud company because
- founder have worked on mRNA technology with his wife for more than 25 years
- A track record: BioNTech Chief Medical Officer Özlem Türeci and her husband, Dr. Sahin, sold their first company, Ganymed Pharmaceuticals, for $1.66 billion before focusing on BioNTech.
- A skaptical partner – Pfizer
How Pfizer Partner BioNTech Became a Leader in Coronavirus Vaccine Race
Small German biotech was a niche player in futuristic cancer treatments—then pivoted when Covid-19 broke out in China
“As with any new technology, mRNA needed to be proven scientifically and, at that time, there was little evidence that RNA technologies could be effective in preventing infectious disease,” Dr. Jansen said. “What intrigued me with the technology was the potential to develop a better flu vaccine for which RNA offered multiple potential advantages to current approaches.”
BioNTech still doesn’t have any approved treatment or vaccine. But Dr. Sahin had been working on mRNA technology with his wife for more than 25 years. The couple, both children of Turkish immigrants who met while working at a cancer clinic, sold their first company, Ganymed Pharmaceuticals AG, for €1.4 billion ($1.66 billion) in 2016.
BioNTech and Pfizer’s influenza jab was due to enter human trials in 2020. But Dr. Sahin’s pivot to Covid-19 in January scrambled the timetable. As the epidemic raged in China—making it a good place to hold vaccine trials—he struck a deal with Shanghai Fosun Pharmaceutical Co., Ltd. to test candidates there.
- 10/15/2020 – Pandemic effects might be
The pandemic has caused the world’s economies to diverge
But its long-term impact will be even more far-reaching
By the end of next year, according to forecasts by the oecd, America’s economy will be the same size as it was in 2019 but China’s will be 10% larger. Europe will still languish beneath its pre-pandemic level of output and could do so for several years—a fate it may share with Japan, which is suffering a demographic squeeze. It is not just the biggest economic blocs that are growing at different speeds. In the second quarter of this year, according to ubs, a bank, the distribution of growth rates across 50 economies was at its widest for at least 40 years.
As our special report this week explains, these adjustments will be immense. The pandemic will leave economies less globalised, more digitised and less equal. As they cut risks in their supply chains and harness automation, manufacturers will bring production closer to home. As office workers continue to work in their kitchens and bedrooms for at least part of the week, lower-paid workers who previously toiled as waiters, cleaners and sales assistants will need to find new jobs in the suburbs. Until they do, they could face long spells of unemployment. In America permanent job losses are mounting even as the headline unemployment rate falls (see article).
As more activity moves online, business will become more dominated by firms with the most advanced intellectual property and the biggest repositories of data; this year’s boom in technology stocks gives a sense of what is coming, as does the digital surge in the banking industry (see our leader on Ant Group). And low real interest rates will keep asset prices high even if economies remain weak. This will widen the gulf between Wall Street and Main Street that emerged after the global financial crisis and which has worsened this year. The challenge for democratic governments will be to adapt to all these changes while maintaining popular consent for their policies and for free markets.
The question-mark is America. For much of the year it got the policy balance roughly right. It provided a more generous safety-net for the jobless and a larger stimulus than might have been expected in the home of capitalism. Wisely, it also allowed the labour market to adjust and has shown less inclination than Europe to bail out firms that are in danger of becoming obsolete as the economy adjusts. Partly as a result, unlike Europe, America is already seeing the creation of many new jobs.
Instead America’s weakness is toxic and divided politics. This week President Donald Trump seemed to ditch talks over renewing its stimulus, meaning that the economy could fall over a fiscal cliff. Critical reforms, whether to redesign the safety-net for a tech-driven economy or to put deficits on a sustainable course, are all but impossible while two warring tribes define compromise as weakness. Covid-19 is imposing a new economic reality. Every country will be called on to adapt, but America faces a daunting task. If it is to lead the post-pandemic world, it will have to reset its politics.
- 10/15/2020 – If vaccine works, golbal economy will improve significantly
Vaccine cooperation, recovery could boost global income $9 trillion by 2025, IMF chief says
- 10/06/2020 – uncertainty on stimulus plan
Despite Trump’s move, markets are still expecting stimulus and a sizable one if Democrats sweep
- Treasury yields have been rising, as it looks more likely Joe Biden will win the White House and help the Democrats sweep in the Senate elections.
- That would put a big infrastructure package in play for early next year, possibly a plus for the economy but negative for bonds since it would bring more debt issuance.
- The market has also been expecting a big fiscal spending package this year, but President Trump put that in doubt when he said his administration will no longer talk to Democrats about the package until after the election.
- 10/05/2020 – will the vaccine come this month? Will public trust this vaccine?
Trump Determined to Get Vaccine Before Election, Reportedly Overrules FDA Guidelines
In his theatrical Monday display of flouting his doctors’ advice and returning to the White House, President Trump’s triumphal videotaped message contained a line that deserves more attention than it received: “The vaccines are coming momentarily.”
Trump has spent weeks hinting that he would like a vaccine to be announced before the election, and also that he distrusts his scientific advisers. Now his administration has overruled the Food and Drug Administration’s proposed vaccine guidelines, according to a report from the New York Times. It is abundantly clear Trump’s political team is overruling its scientists in order to rush through the approval of a vaccine before the election.
- 10/05/2020 -chapter 11 has surged by 200% due to Cv-19
Fewer Americans have filed for bankruptcy in 2020 than in 2019
But the reasons why tell a depressing tale

TO SAY THAT the pandemic has been hard for the American economy would be putting it mildly. The unemployment rate, which stood at just 3.5% in February, is now 7.9%; there are 10.7m fewer jobs today than there were six months ago; a quarter of the workforce is working from home. You might expect such dismal economic conditions to be accompanied by a spike in bankruptcies. But so far this year, bankruptcy filings are down by 27%.
In a new paper, researchers at the University of Illinois, Brigham Young and Harvard collected data from online court filings to estimate the impact of the covid-19 pandemic on bankruptcies. They found that, unlike past business cycles, when worsening economic conditions led to more bankruptcies, this downturn has actually yielded fewer. Filings were down by nearly 140,000 in the first eight months of 2020, compared with the same period in 2019. Personal bankruptcies were down by 28%; business bankruptcies by 1% (see chart).
Though this seems encouraging at first glance, the details are less rosy. Take business bankruptcies. The authors note that filings under Chapter 7, a part of America’s bankruptcy code used mainly by smaller firms wishing to liquidate outright and sell their assets to pay creditors, have fallen by 13%, year on year. But the decrease in Chapter 7 filings has been largely offset by a 35% jump in filings under Chapter 11, the form of bankruptcy covered in the business pages of American newspapers involving bigger companies aiming to restructure their debts and continue operating. Chapter 11 filings by firms with more than $50m in assets have surged by nearly 200%.
The authors argue that small companies have had a harder time securing access to the bankruptcy system during the pandemic, which has delayed filings. Social-distancing measures have forced bankruptcy courts to conduct hearings by telephone or video conference, rather than in person. Some courts have shut down entirely. The pandemic has also made it harder for business owners to avail themselves of legal services. And whereas big companies turn to bankruptcy as a source of protection, small firms view it as a last resort.
Consumer bankruptcies, meanwhile, are down by more than a quarter on the year. Filings under Chapter 13 of the code, which allows individuals to keep their property and commit themselves to a repayment plan, have decreased by 41%. Chapter 13, as it happens, is used mainly by wealthier people and homeowners. These households, the authors argue, may have been less affected by the downturn, and were aided by government interventions such as the mortgage moratorium mandated by the CARES Act (the $2.2trn coronavirus-relief package passed into law in March). Consumer filings under Chapter 7, typically used by people with lower incomes and fewer assets, fell by 20% between January and August. Both types of filing fell by more in states with high unemployment than in those with low unemployment: further evidence that, in a crisis, those who are already worst-off are often hit the hardest.
- 09/04/2020 – Small chance of vaccine in end of Oct
Trump claims coronavirus vaccine could be delivered next month
The president denied the timing is tied to the November election
- 09/04/2020 – Playing derivatives drive tech stock high?
SoftBank’s Bet on Tech Giants Fueled Powerful Market Rally
Japanese conglomerate led by billionaire Masayoshi Son placed billions in options bets on fast-rising tech stocks
How Options-Market Amateurs Might Have Tripped Up Big Tech
Retail investors have started to buy derivatives of Silicon Valley stocks rather than the underlying assets, increasing the risk of sudden sell-offs
- 09/03/2020 – Biden also wants more stimulus money, not sure it will be legislated by Congress/senate or not
As Stimulus Package Stalls, Biden Has $4 Trillion Economic Plan
- 09/02/2020 – Trump admin pushes vaccine to be ready before election day
Trump Administration Asks States to Be Ready for Vaccine by November
CDC sent letter to governors asking them to speed approval of distribution centers by Nov. 1, just before Election Day
WASHINGTON—The Trump administration is asking states to speed up approval for vaccine distribution sites by Nov. 1, the latest sign the federal government is eager to get a vaccine out before the end of the year.
Centers for Disease Control and Prevention Director Robert Redfield urged state governors to remove barriers to building permits for distribution sites for use by McKesson Corp. and the drug wholesaler’s subsidiaries, according to an Aug. 27 letter. The Dallas-based company has a deal with the federal government to distribute a coronavirus vaccine when it becomes available.
“CDC urgently requests your assistance in expediting applications for these distribution facilities, and, if necessary, asks that you consider waiving requirements that would prevent these facilities from becoming fully operational by Nov. 1, 2020,” read the letter from Dr. Redfield to the states.
- 09/02/2020 – all news on CV-19 from WSJ
The Coronavirus Crisis
The Wall Street Journal’s coverage of the pandemic, from the latest news to deeper looks into how the disease is affecting life and business.
- 08/31/2020 – implication of emergency release of vaccine
Here’s how the U.S. could release a COVID-19 vaccine before the election—and why that scares some
What’s the traditional vaccine approval pathway?
After initial laboratory and animal tests, vaccines enter phase I human trials that typically have about 20 to 100 people and primarily analyze safety and immune responses. Phase II studies are larger versions of phase I trials. Phase III studies attempt to determine whether a vaccine works by comparing people who receive it with those who are given a placebo shot and, over several months or years, seeing how many in each group get infected. For COVID-19 vaccines these trials involve anywhere from 10,000 to 60,000 people and will need a total of about 150 cases of disease to determine whether a candidate works. Once the trial endpoints are met, a vaccine developer seeking FDA approval would file a biologics license application; VRBPAC would review the data at a public meeting, then vote on whether the vaccine should receive approval—a recommendation FDA normally follows. The approval process, which involves inspecting the vaccine’s manufacturing plants, can often take 1 year.
How does an EUA work?
An EUA in the United States, and similar regulatory pathways in many countries, allows use of an unlicensed vaccine outside of a clinical trial. The EUA could stipulate the use of the vaccine in a limited population, for example, health care workers or the elderly. Or it could be for the general population. An EUA offers liability protections to vaccinemakers, and it remains in effect as long as there is a public health, military, or national security emergency. When the emergency ends, so does the approval.
What safety and efficacy evidence would FDA require before issuing an EUA?
FDA issued a “guidance for industry” in June that says any emergency decision on a COVID-19 vaccine would be based on factors such as “the target population, the characteristics of the product, [and] the preclinical and human clinical study data.” The guidance specifies that FDA will only approve an EUA for a vaccine that has at least 50% efficacy. But estimates of efficacy have error bars of sorts; for a COVID-19 vaccine, FDA wants 95% confidence that efficacy is no lower than 30%. The decision to consider an EUA request would likely be based on data reviewed by the independent boards, set up by the vaccine’s sponsors or clinical trial investigators, that monitor safety and efficacy during the study. (A Financial Times story on 30 August quoted Hahn saying an EUA could be issued prior to the completion of a phase III study. A senior official at the Department of Health and Human Services confirms Hahn was referring to a potential EUA request prompted by routine, early looks at phase III data by the independent boards tasked with monitoring safety.)
What harm could an EUA do?
Public Citizen, a public advocacy group, has argued that regardless of whether a COVID-19 vaccine is effective, an EUA could fuel existing vaccine hesitancy. “The ‘logic’ of saving several months by a faster but riskier EUA pathway will surely be outweighed by the loss in public confidence in the vaccine, accompanied by decreased willingness to be vaccinated,” Public Citizen warned in a 6 August letter to Marks and his superiors. An EUA for a vaccine might also make it more difficult to recruit people for clinical trials of that vaccine and others, because participants might not want to take the risk of receiving a placebo when they can get a shot of a product that’s authorized for use.
What if the vaccine doesn’t work well or causes harm?
Vaccines go into healthy people, so putting them into use before fully assessing their risks and benefits is a bigger gamble than issuing an EUA for an experimental treatment for someone already ill. If a hastily approved COVID-19 vaccine candidate proves ineffective or has serious side effects, confidence in what many see as the best hope to ending the pandemic could plummet. The Solidarity Vaccines Trials Expert Group of the World Health Organization (WHO) argued in an editorial published in The Lancet yesterday that a weakly effective vaccine could actually worsen the pandemic if it induced authorities to relax control measures, such as mask wearing, or if vaccinated people believed they were immune and increased their risk-taking behavior.
Has an EUA ever been used for a vaccine?
Yes. In 2005 FDA granted an EUA for an anthrax vaccine for people who the military determined were at high risk of attack from anthrax used as a biological weapon. The episode provoked lawsuits claiming there was no evidence that the vaccine, which the military required soldiers to get, worked against the type of inhalational anthrax used in bioweapons. A judge ruled in favor of the plaintiffs, but by then the vaccine had become voluntary.
What’s the difference between FDA’s expanded access program and an EUA?
Typically, expanded access, also called compassionate use, covers treatments, not vaccines, in the United States. It’s for individuals who have a life-threatening condition and no alternatives or for small groups of sick people when a treatment has promising evidence but efficacy has not been proved yet. Anyone who receives the experimental medicine signs an informed consent form, and institutions that provide it have to seek permission from FDA, submit a protocol, report adverse events, and do continued safety monitoring. An EUA eliminates these requirements. FDA allowed nearly 100,000 people to receive convalescent plasma through expanded access—an unusually large instance of compassionate use—but last week granted an EUA that proponents said would cut paperwork. The Democratic Republic of the Congo used its own expanded access regulation to allow more than 300,000 people to use an unlicensed Ebola vaccine.
Does Europe have a similar emergency approval process?
The European Medicines Agency (EMA) can issue “conditional approval” for a vaccine during a pandemic. Under a rolling-review process, companies continue to submit data as they become available. The United Kingdom, which will be leaving EMA’s authority because of Brexit, today issued a consultation for public comment on how its regulatory agency might issue its own temporary authorization of an unlicensed COVID-19 vaccine.
How did China and Russia speed approval of their COVID-19 vaccines?
China on 25 June gave CanSino a 1-year approval to use its COVID-19 vaccine in the Chinese military, although there is no evidence beyond statements by company officials that anyone has received it. On 22 July, China also allowed Sinopharm’s China National Biotec Group Company to give its COVID-19 vaccine to health care workers, customs workers, and others in “high-risk” professions. Both vaccines are still in phase III efficacy trials. CanSino also reportedly is in discussion with regulators in Pakistan and unnamed Latin American countries about early approval of its vaccine.
Russia’s Gamaleya Research Institute of Epidemiology and Microbiology in Moscow on 11 August received a “registration certificate” to give a COVID-19 vaccine to what a Ministry of Health spokesperson described as “a small number of citizens from vulnerable groups,” including medical staff and the elderly. Dubbed Sputnik V, a clear reference to the U.S.-Soviet space race, the product is billed as “the first registered COVID-19 vaccine.” The registration says it cannot be used widely until after 1 January 2021, but President Vladimir Putin said, “I hope we can start a massive release of this vaccine soon.”
Many countries do not have strong regulatory agencies. How do they decide whether to use a COVID-19 vaccine that is not licensed?
WHO has what it calls an Emergency Use Listing, which many low- and middle-income countries have relied on in the past. “We can give a benefit/risk decision on a product and specify the conditions under which it should be used,” says Emer Cooke, director of WHO’s regulation of medicines and other health technologies. “We act like a regulatory body, but we’re not a regulatory body.” Cooke, who recently was elected to head EMA later this year, says their job is especially complex now because of the flood of COVID-19 vaccine candidates and the intense pressure to find one that is safe and effective. “I think we are seeing more political influences now than we would normally see,” she says.
- 08/31/2020 – 33 candidates are in clinical trial. 9 candidates are in phase III. The earliest time to file regulatory clearance is in Oct
Covid-19 Vaccines: What’s Coming and When?
As countries and companies race to produce a safe and effective coronavirus vaccine, here’s a guide to the front-runners
Some 170 Covid-19 vaccines are in development around the world, according to the World Health Organization, each one promising to protect people from the deadly coronavirus and allow them to go back to work and school. 33 candidates are in clinical trial. 9 candidates are in phase III.
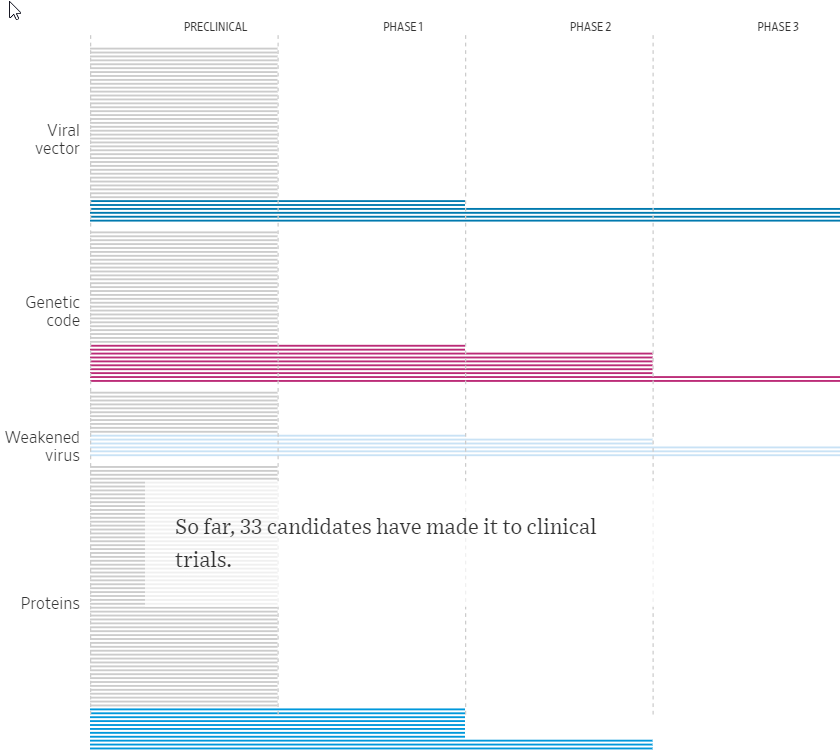
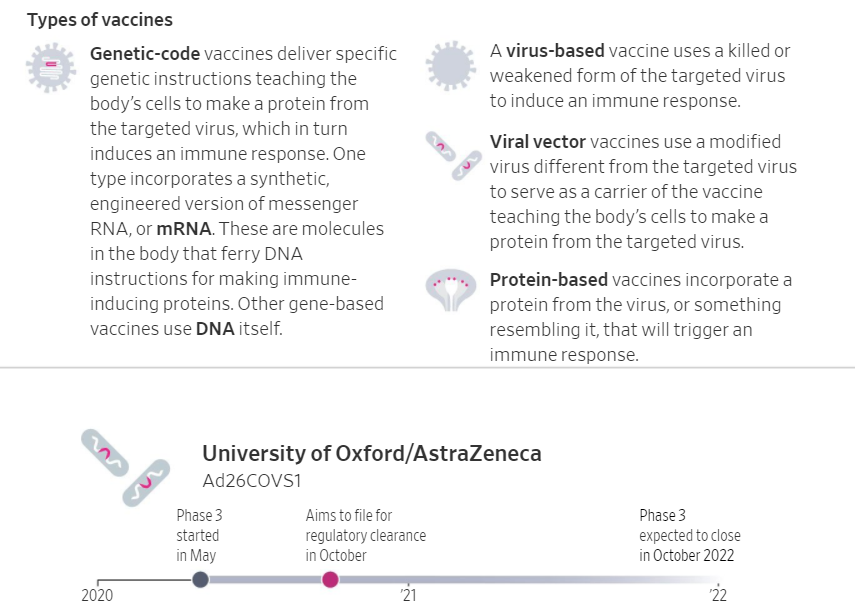
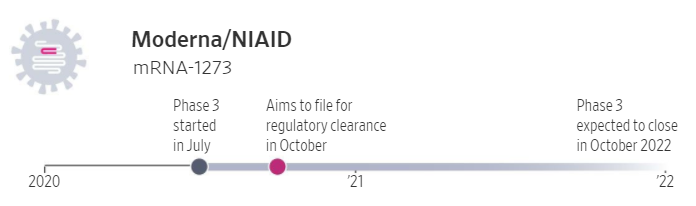
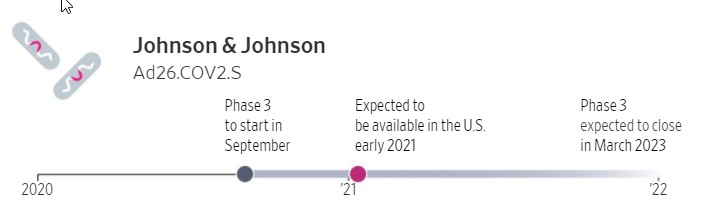
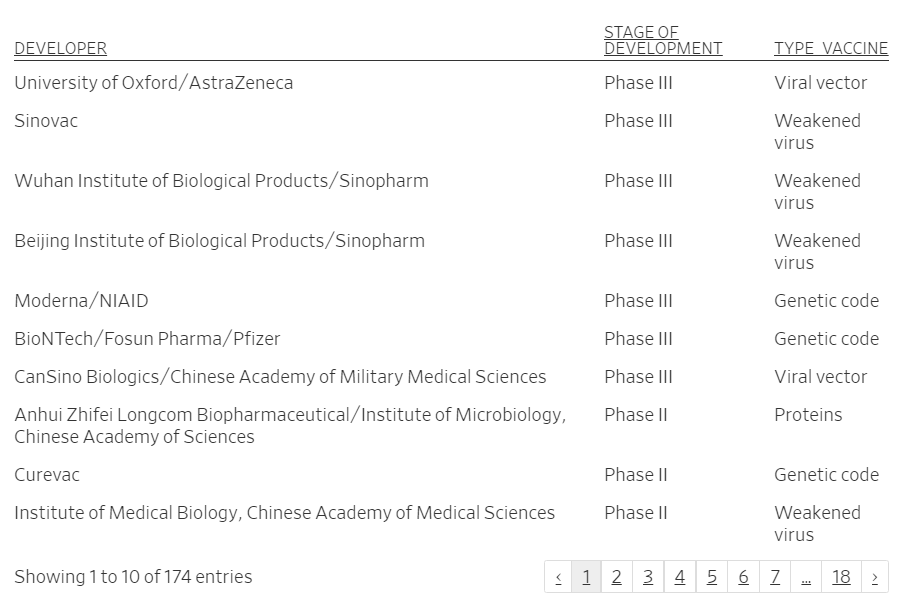
- 08/31/2020 – So sole CV death is only 9000? “For 6% of the deaths, COVID-19 was the only cause mentioned.”
Comorbidities
Table 3 shows the types of health conditions and contributing causes mentioned in conjunction with deaths involving coronavirus disease 2019 (COVID-19). For 6% of the deaths, COVID-19 was the only cause mentioned. For deaths with conditions or causes in addition to COVID-19, on average, there were 2.6 additional conditions or causes per death. The number of deaths with each condition or cause is shown for all deaths and by age groups. For data on comorbidities,
- 08/28/2020 – two sides remain at a ‘tragic impasse’
Pelosi says virus relief talks will resume when GOP agrees to $2.2T price tag: ‘We’re not budging’
The top Democrat said the two sides remain at a ‘tragic impasse’
- 08/28/2020 – no Covid-19 relief bill until after September? more volatility is coming in end of Sept?
Mark Meadows predicts no Covid-19 relief bill until after September
White House Chief of Staff Mark Meadows said Wednesday he is not optimistic about reaching a new coronavirus relief deal before the end of September, predicting House Speaker Nancy Pelosi will use the government funding cliff at the end of next month as leverage to strike a deal on pandemic aid.
Speaking with POLITICO’s Jake Sherman and Anna Palmer, Meadows said his staff had reached out to Pelosi’s office Tuesday but added that he does not anticipate a response. The White House chief of staff said lawmakers from both parties have privately expressed to him a desire to make progress on coronavirus relief. The hold up, Meadows said he suspects, is that Pelosi is holding back her party’s rank and file in order to secure more Democratic priorities in any legislation.
background knowledge: United States fiscal cliff
- 08/27/2020 – Fed is determined to push economy higher and higher
Fed Approves Shift on Inflation Goal, Ushering in Longer Era of Low Rates
Chairman Jerome Powell says central bank has changed how it views trade-off between lower unemployment and higher inflation
- 08/27/2020 – good initial sign from Moderna on CV-19 vaccine
Moderna Says Covid-19 Vaccine Shows Signs of Working in Older Adults
Results from preliminary study indicated vaccine produced similar levels of antibodies in seniors as in younger subjects
- 08/20/2020 – FDA will not yield to political pressure and tries to be fair
Exclusive: Top FDA official says would resign if agency rubber-stamps an unproven COVID-19 vaccine
A top U.S. health regulator who will help decide the fate of a coronavirus vaccine has vowed to resign if the Trump administration approves a vaccine before it is shown to be safe and effective, Reuters has learned.
He added that he would equally object if someone sought political gain by holding up approval of a vaccine that was shown to work, and that was safe.
- 08/20/2020 – It seems like negotiation of next stimulus plan will not start until after Labor Day
Pelosi rejects call to extend coronavirus unemployment relief benefits with smaller bill
“I don’t think, strategically, it’s where we should go right now,” the House Speaker said.
Pelosi said she did not expect any bill to be passed within the next few days.
“I don’t think the timing is for us to do it right now, because, imagine, the Republicans could take that into the Senate, put poison pills all over it. And it’s hard to vote against extending unemployment benefits,” she said. “And, again, I think, overwhelmingly, our members — who would not want to extend unemployment benefits? As I say, it’s something I fully support, and the stabilization, but not necessarily in the negotiation.”
Pelosi called the House back into session to vote Saturday on legislation related to the U.S. Postal Service. Lawmakers will vote on whether to block any changes to its operations ahead of the 2020 presidential election.
- 08/20/2020 – news about a Covid-19 vaccine is heating up again. The flurry of news serves as something of a warm-up for the coming months, where vaccine developments could drive stock prices across the market. The external advisory committee would likely need to weigh in before the FDA would issue an emergency-use authorization. And an Oct. 22 meeting would leave time for a vaccine to be approved before the election. Watch out the news
A Tidal Wave of Vaccine News Is Hitting the Market
In one notable development, BioNTech (BNTX) and Pfizer (PFE) published data on the experimental vaccine the companies are testing in Phase 2/3 trials. It is the second of two versions of the vaccine that the company tested in Phase 1 trials. A paper published on a non-peer-reviewed preprint server says that it appears safer than the previous version.
Also on Thursday, The Wall Street Journal reported that Johnson & Johnson (JNJ) plans a 60,000 person trial of its Covid-19 vaccine by late next month. Phase 3 trials launched by Pfizer and Moderna (MRNA) aim to enroll 30,000 people. The Journal said it isn’t clear why the Johnson & Johnson trial aims to be twice as large.
Meanwhile, in a call with reporters Thursday morning, a Food and Drug Administration official said that the agency’s external vaccine- advisory panel might meet on Oct. 22 to discuss a Covid-19 vaccine, according to Bloomberg. The official did not say which vaccine the panel would discuss, but Pfizer has said it hopes to have Phase 3 data on its vaccine by then.
The external advisory committee would likely need to weigh in before the FDA would issue an emergency-use authorization. And an Oct. 22 meeting would leave time for a vaccine to be approved before the election. Investors increasingly expect approval by then, despite worries from former FDA officials that the timing could undermine trust in the product.
All that comes after CureVac (CVAC) said early Thursday that it was in talks with the European Commission to sell it 225 million doses of its experimental Covid-19 vaccine, sending shares of the newly public stock up 14.7%.
Shares of BioNTech were up 2% on Thursday at midday, while shares of Pfizer were up 0.6%. Shares of Moderna were down 1.1%, and shares of fellow Covid-19 vaccine developer Novavax (NVAX) were off 0.4%. Stock in Johnson & Johnson was flat,.
The BioNTech and Pfizer data was particularly promising. The companies said in late July that they were beginning a Phase 2/3 trial of BNT162b2, one of two similar versions of the vaccine they had developed. But the companies had only released Phase 1 data on the other version, called BNT162b1. The paper out Thursday outlines the results of the BNT162b2 test, and says that while the immune responses elicited by the two vaccines were similar, the second version had a better safety profile.
“The data set presented here guided our decision to advance BNT162b2… into the Phase 2/3,” the paper reads. “The primary consideration driving this decision was the milder systemic reactogenicity profile of BNT162b2, particularly in older adults, in the context of comparable antibody responses elicited by both candidate vaccines.”
The flurry of news serves as something of a warm-up for the coming months, where vaccine developments could drive stock prices across the market.
- 08/19/2020 – the negotiation on stimulus plan might restart right after Labor Day 09/07. The Senate is due to return from recess on Sept. 8 and the House on Sept. 14.
As White House pushes ‘skinny’ COVID-19 bill, Democrat sees September action
WASHINGTON (Reuters) – The White House on Wednesday pushed for Congress to take up a narrow coronavirus economic relief bill that Democrats have long rejected, while a leading Senate Democrat said real action may come soon after the Sept. 7 U.S. Labor Day holiday.
Democratic Senator Tim Kaine said that he does not expect the White House to get serious about negotiations until after next week’s Republican presidential election convention, where he expects Republicans to tout President Donald Trump’s executive orders on coronavirus relief.
“Once we get out of the Republican convention, the week before Labor Day, you’re going to see serious negotiations restart. And that means we could do something possibly right after Labor Day, when we return,” Kaine said in an online interview with Politico.
The Senate is due to return from recess on Sept. 8 and the House on Sept. 14.
- 08/18/2020 – The emergent fund will be used up in weeks, so Dem and Rep need to make decision on the stimulus plan. Trump wins the battle temporarily
Mnuchin Says Stimulus Talks Remain Stalled
Treasury secretary suggests Pelosi may be open to resuming talks when House convenes to take up Postal Service bill
Mr. Mnuchin on Tuesday gave little indication that the battle lines have budged, saying, “I really don’t know,” when asked why a deal seems so far off.
“We started with a trillion dollars. We agreed to increase that in several areas in an effort to compromise,” he said. “They didn’t come down. They never made us a proposal at two trillion; they never gave us a line-by-line counter.”
- 08/14/2020 – retail sales are back to all-time high, still need stimulus plan to sustain it?
Retail sales bounced to an all-time high, but lack of stimulus from Congress puts gains at risk
- Retail sales were up 1.2% to a record level in july, and were stronger than expected when excluding vehicle sales.
- But economists say the outlook for August is not nearly as positive, and sales will be flat or lower because supplemental unemployment benefits paying individuals $600 a week has run out.
- They say unless some amount of payments are restored, and a stimulus package is approved, the economy will struggle.
- 08/14/2020 – good incentive to push vaccine companies to make vaccine faster
Vaccine makers including Moderna must hit U.S. timing goals for full payments
The United States is tying payments for COVID-19 vaccines to timing milestones for production and approval, according to public documents and a Trump administration official, putting pressure on drugmakers including Moderna Inc (MRNA.O) to meet ambitious targets.
- 08/13/2020 – so this might not happen before election day?
Top U.S. health official says approval of COVID vaccines unlikely before November
(Reuters) – Any potential COVID-19 vaccine backed by the Trump administration’s “Operation Warp Speed” program is unlikely to receive a green light from regulators any earlier than November or December, given the time needed for a large-scale clinical trial, the National Institutes of Health director said on Thursday.
- 07/20/2020 – We might know by the end of August whether or not the vaccine works. And emergency approval before further trials are conducted could happen as early as October.
Trials of a vaccine and new drug raise hope of beating covid-19
The latest tests with Oxford University’s vaccine, and interferon beta, look promising
The Oxford vaccine is furthest ahead with such trials. A 10,000-patient trial in Britain has almost finished recruiting, and recruitment is rising quickly for a trial in Brazil. Trials in South Africa have just started, and another will begin in America in the coming weeks. If everything goes well it could be clear to researchers by the end of August whether or not the vaccine works. When the results of the first of these trials are available, assuming they are positive, regulators will have to decide whether there are enough data to allow for some sort of emergency approval before further trials are conducted. That could happen as early as October.
- 08/11/2020 – We might can get the first signal of vaccine efficacy by Oct
BioNTech and Pfizer Hope to Have Late-Stage Coronavirus Vaccine Results by October
On Tuesday, BioNTech (NASDAQ:BNTX) confirmed what its big pharma partner, Pfizer (NYSE:PFE), had already let out of the bag: The companies hope to have phase 3 results for their coronavirus vaccine candidate by October.
In late July, the duo selected the best vaccine from its early candidates to take into phase 2b/3 development and started the study shortly thereafter. Once the clinical trial is fully enrolled and all of the approximately 30,000 subjects have received the vaccine and placebo, it’ll take another 21 days before the subjects will get their booster shots.
Assuming the drugmakers wrap up enrollment in the next week or so, the last booster for the final patient would be given in the middle of September. That doesn’t give much time before the self-imposed expectations for data in October.
Of course, there are some caveats that could make the short timeline plausible. It isn’t clear, for instance, if BioNTech and Pfizer think they need data from all 30,000 patients to gain regulatory authorization or approval. If the difference between subjects given the coronavirus vaccine and those given placebo are strong enough, it’s possible regulators could give a thumbs up based on data from a subset of patients treated early in the study.
It also isn’t entirely clear if the companies plan to file with data showing the vaccine creates antibodies, which can be generated fairly quickly after the booster shot, or whether regulatory agencies will require companies to show a difference in the rate patients develop COVID-19 after treatment with the vaccine or placebo.
- 08/11/2020 – Trump and Dem open to talk more
Trump, Democrats Open to Restarting Coronavirus Talks Despite Stalemate
White House wants to keep down size of relief package; Democrats say more aid is needed
Trump administration officials and Democratic leaders urged each other to return to the negotiating table to craft a broad coronavirus package after President Trump issued executive actions on jobless aid and other relief over the weekend.
- 08/10/2020 – PPP is a great job and economy booster, hope it can be extended
PPP responsible for ‘majority’ of jobs created since May, SBA administrator says
There’s roughly $135B remaining in the fund
A relief program that threw a critical lifeline to small businesses during the coronavirus pandemic is responsible for a “majority” of the jobs created since May, according to Small Business Administration Administrator Jovita Carranza.
The $670 billion Paycheck Protection Program, created when Congress passed the CARES Act at the end of March, officially closed to new applicants on Saturday.
- 08/10/2020 – Fed continues to support economy by buying more blue-chip and junk bonds and making Main Street loans
The Fed bought more blue-chip and junk bonds, and has started making Main Street loans
- The Federal Reserve continued its purchases of corporate bonds in July, buying up both blue-chip and junk issues.
- In addition, the central bank made its first move through the Main Street Lending Program, though the loans totaled just $92 million for a program that can lend $600 billion.
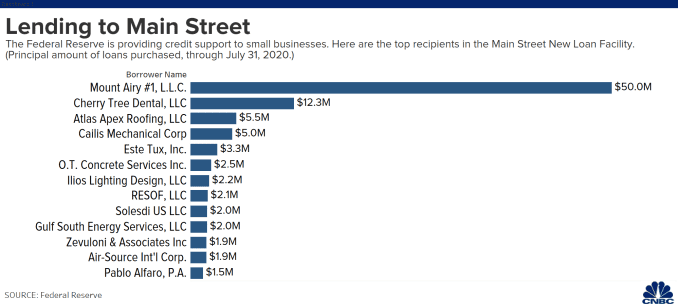
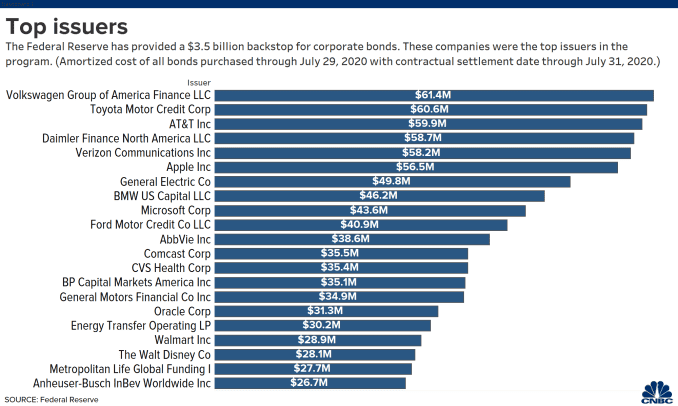
The central bank’s biggest position on the exchange-traded front remains the iShares iBoxx US Dollar Investment Grade Corporate Bond fund, with share purchases worth $2.47 billion. The iShares ETF family is owned by asset management giant BlackRock, which is helping the Fed run the credit facilities.
In addition, the Fed stepped up its buying of junk bonds, purchasing $331 million worth of the iShares iBoxx High Yield Corporate Bond ETF, a move up from June’s buying of $274.6 million. It also continued its purchases of bonds that were low-level investment grade heading into the pandemic and then were downgraded. It bought $34.6 million of the VanEck Vectors Fallen Angel High Yield Bond fund.
The Fed has yet to make any purchases on the primary market.
Just one loan went out through the municipal lending facility, to the state of Illinois for $1.2 billion.
- 08/10/2020 – Trump’s admin is willing to compromise reasonably
Mnuchin is open to restarting virus relief talks: ‘We’re prepared to put more money on the table
- Treasury Secretary Steven Mnuchin said the White House is open to resuming coronavirus aid talks with Democrats and putting more relief money on the table to reach a compromise.
- He did not give a timeline for when the sides would resume discussions, but said he hoped they could reach a deal this week if Democrats made a fair offer.
- President Donald Trump took executive action to try to extend pandemic aid after talks collapsed on Friday.
- 08/10/2020 – Trump admin is playing hard ball on deal making. Pelosi is willing to compromise
Mnuchin Says White House Won’t Accept Unreasonable Coronavirus Stimulus Deal
Talks deadlocked, Treasury secretary says ‘If we can get a fair deal, we’ll do it this week’
Mnuchin says Dems ‘willing to compromise’ on coronavirus stimulus package as negotiations stalled
‘If we can get a fair deal, we’ll do it this week,’ Treasury secretary says
Treasury Secretary Steven Mnuchin said Monday that he believes Democrats are “willing to compromise” on a fourth coronavirus stimulus package, saying that if there is a “fair deal,” the Trump administration will “do it this week.”
Mnuchin, during an interview on CNBC Monday morning, said he wouldn’t comment on the “specifics of logistics of negotiations” because he does “not think that’s helpful.”
“There is a deal to do if Democrats are reasonable and want to compromise,” Mnuchin said. “And if the attitude is, we’d rather give you nothing than agree on things, then we’re not gonna get a deal.”
“But I heard [Nancy] Pelosi over the weekend,” Mnuchin said. “They’re willing to compromise, so if we can get a fair deal, we’ll do it this week, but the president needed to take action.” He added: “He’s not gonna sit around. Meadows and I reported back to him that we’re going nowhere, and that’s why he took action.”
- 08/09/2020 – So Trump is still waiting for a better deal from democrats before the end of August?
Why Trump’s Measures Are More Likely to Have a Political Effect Than an Impact on the Economy
With negotiations in Congress over a new aid package at an impasse, President Donald Trump on Saturday signed four executive orders—including one to extend enhanced unemployment insurance. The orders, however, have big caveats and aren’t a substitute for a fiscal deal.
The executive orders also include deferrals of payroll tax withholdings for certain workers, deferrals of student loan payments, and action to minimize certain evictions.
The president’s actions are likely to have more of a political effect—they should increase the urgency for negotiators to reach a broader fiscal deal—than an economic impact.
Key for the U.S. economy has been the fate of the extra $600 a week that millions of newly unemployed workers stopped receiving at the end of July. The enhanced federal and state unemployment benefits have propped up household income during the pandemic, totaling $1.4 trillion in June from pre-pandemic levels of around $28 billion and preventing the U.S. economy from falling into an even deeper hole.
While Trump’s order partially extends the extra jobless payments by $400 a week, there are important caveats. First, the federal government is funding only 75% of the extension, paid for from the Disaster Relief Fund. The order says states must fund the rest, and it appears that if a worker’s state is unable to do so, the worker receives no part of the enhanced payment. This catch is particularly important given states’ increasingly dire fiscal situations as tax revenue drops because of the pandemic.
Second, the executive order says the extra weekly benefits would be available until Dec. 6, a month after the presidential election, or until the disaster fund’s balance drops to $25 billion. But as the fund currently has a balance of about $70 billion, economists at Goldman Sachs say in reality the benefits would probably last only a month.
Goldman highlights another meaningful stipulation when it comes to the payroll tax deferral. Trump’s order directs the Treasury Department to defer withholding of payroll taxes for employees making less than $4,000 every two weeks (that’s $104,000 a year), from Sept. 1 through Dec. 31, 2020. The order appears to defer only the employee-side 6.2% Social Security tax, as the Cares Act already deferred employer-side payroll taxes through the end of the year.
But only Congress can reduce or eliminate the payroll tax. For this order to have a meaningful effect, Golman economists say, employees would need to be confident that Congress would eventually pass a tax cut to forgive the deferred tax liabilities. Otherwise, workers would face a substantial tax bill in 2021 and might either voluntarily withhold extra taxes or save the extra pay to cover the expected tax bill, the economists say.
Goldman points to a unilateral tax withholding change in 1992 to predict the potential response of workers and consumers. About a month after the change, Goldman says, surveys suggested that more than half of consumers either changed their withholding to offset the policy, saved the money or paid down debt. The Congressional Budget Office later estimated that about half of the potential reduction in tax withholding was offset by individuals who voluntarily raised their withholding to offset the change.
Considering the catches that come with these directives, it appears the executive orders may have more of an effect on motivating Republicans and Democrats to reach a deal than on buffering the economy. The executive orders create new deadlines around the end of the month—the payroll tax deferment is scheduled to take effect Sept. 1, and it appears the funds behind the extended unemployment benefits might run dry in early September—potentially providing a new incentive for Congress to agree to a new, fuller package in August.
“We suspect that the possibility of unilateral action might focus minds on Capitol Hill and increase the odds of an agreement,” Goldman Sachs says.
- 08/07/2020 – The FDA has said it would authorize a coronavirus vaccine so long as it is safe and at least 50% effective
Dr. Anthony Fauci says chance of coronavirus vaccine being highly effective is ‘not great’
- White House coronavirus advisor Dr. Anthony Fauci that the chances of scientists creating a highly effective vaccine — one that provides 98% or more guaranteed protection — for the virus are slim.
- Scientists are hoping for a coronavirus vaccine that is at least 75% effective, but 50% or 60% effective would be acceptable, too, he said.
- The FDA has said it would authorize a coronavirus vaccine so long as it is safe and at least 50% effective.
- 08/07/2020 – Trump admin does not yield to Pelosi’s bargain because they plan to use executive power
Mnuchin rejects $2T coronavirus stimulus offer from Democrats: ‘That’s a non-starter’
Mnuchin called the proposal for each party to give $1 trillion a ‘non-starter’
Treasury Secretary Steven Mnuchin rejected an offer from Democratic leaders for a roughly $2 trillion coronavirus relief package amid growing concerns that negotiations between the two parties are on the brink of collapsing.
“That’s a non-starter,” Mnuchin said Friday.
House Speaker Nancy Pelosi proposed the $2 trillion price tag during a closed-door meeting on Thursday, an attempt to reconcile the two party’s drastically different aid proposals. Democrats offered to reduce their $3.4 trillion aid package by $1 trillion if Republicans would agree to raise their estimated $1 trillion proposal by the same amount.
Affter nearly two weeks of talks that have yielded no substantial progress, President Trump has threatened to act unilaterally and issue executive orders to continue expanded unemployment benefits, reinstate an eviction moratorium, cut payroll taxes and continue a suspension of student loan repayments.
However, some of his proposals would likely face legal challenges. The Constitution gives Congress the power of federal spending, meaning that Trump does not have the legal authority to issue executive orders allocating how much money should be spent on the pandemic.
- 08/06/2020 – this might rock the tech world and sink Nasdaq/SP500. “It would pose a severe test for U.S. companies if U.S. chips, software, and terminal equipment become irrelevant to the Chinese market.”
Chinese state media slams ‘madness’ of U.S. tech purge
SHANGHAI (Reuters) – Washington’s plan to ban certain technologies of Chinese origin is a sign of “madness” in U.S. Secretary of State Mike Pompeo, China’s state-backed tabloid Global Times wrote in an editorial on Thursday.
“Pompeo has uttered anti-China remarks almost every day, and constantly played tricks to intensify conflicts between China and the U.S., and display Trump administration’s toughness toward China,” the editorial read.
The U.S. State Department on Wednesday published an expanded update of a plan called the “Clean Network” calling for telecom companies, cloud service providers, and mobile apps of Chinese origin to be kept out of the United States.
“From the long-term perspective, it’s incredible that the U.S. information industry could totally detach from the Chinese market,” the Global Times wrote.
“It would pose a severe test for U.S. companies if U.S. chips, software, and terminal equipment become irrelevant to the Chinese market.”
If enacted, the plan would mark an escalation in the ongoing tech spat between the United States and China.
additional news
Trump advisers urge delisting of U.S.-listed Chinese companies that fail to meet audit standards
- 08/05/2020 – Trump is pushing the deal
President Trump weighs executive order imposing stimulus
The constitutionality of such a move in unclear
President Trump is considering an executive order that would unilaterally impose a stimulus plan and include a suspension of the payroll tax, an extension of federal unemployment benefits, an eviction moratorium and another round of individual stimulus checks.
The constitutionality of such a move in unclear. And some people tell FOX Business Trump may be using this as a negotiating tactic to bring Democrats to the table and hammer out a compromise. Either way, Trump is floating this idea with key advisers.
- 08/04/2020 – Senate GOP is ready to compromise
- Mitch McConnell said he is “prepared to support” a coronavirus relief agreement when Democrats and the White House reach one.
- Democrats and the Trump administration are set to meet again Tuesday afternoon as they try to strike a pandemic aid deal.
- The sides appear to have moved toward a consensus on small business aid and stimulus checks but remain divided over unemployment insurance, aid to state and local governments and rent, mortgage and food assistance.
- The $600-per-week federal unemployment benefit and a moratorium on evictions from federally backed housing have expired.
- 08/03/2020 – Anthony Fauci, the top U.S. infectious diseases expert, called them “almost a sure bet” against COVID-19.
Next big COVID-19 treatment may be manufactured antibodies
As the world awaits a COVID-19 vaccine, the next big advance in battling the pandemic could come from a class of biotech therapies widely used against cancer and other disorders – antibodies designed specifically to attack this new virus.
There are also questions about when in the course of the illness it might be best to employ these new weapons.
“Giving an antibody later on after infection might not be that helpful, said Florian Krammer, microbiology professor at New York’s Icahn School of Medicine. “Given early, they probably work well.”
- 08/03/2020 – not a confident vote for Moderna’s vaccine
Moderna CMO sells shares as final vaccine trials begin, raising concerns
CEO Stéphane Bancel has also cashed out on shares in recent months.
- 08/03/2020 – an October surprise for President Trump is not garuanteed
Scientists Worry About Political Influence Over Coronavirus Vaccine Project
Operation Warp Speed has moved along at a rapid clip. But some people involved in the process fear pressure to deliver an October surprise for President Trump.
- 08/02/2020 – It seems like insiders of Moderna are not that confident on the vaccine, at least they want to make boatload of money before results of phase 3 trial.
Moderna CMO sells shares as final vaccine trials begin, raising concerns
CEO Stéphane Bancel has also cashed out on shares in recent months.
As Moderna begins a late-stage trial of its coronavirus vaccine, chief medical officer Tal Zaks sold almost all his shares in the company, according to a report filed to the US Securities and Exchange Commission, raising concerns about his trust in the vaccine, according to Globes. While Zaks and other Moderna officials have already been cashing out on shares for the past few months, they’ve increased the sales of shares since reports were published on a successful test of the vaccine earlier in July.
In general, when stakeholders believe in their product, they increase their shares in order to increase confidence in the market. The move by Moderna officials to do the opposite raised concerns about the company, especially considering that Zaks, who sold almost all of his shares, is on the scientific side of the company, according to Globes.
Zaks still has tens of thousands of dollars worth of options in the company. CEO Stéphane Bancel has also cashed out on shares in recent months. Share sales by CEO Stéphane Bancel, his children’s’ trust and companies he owns amount to about $21 million between January 1 and June 26. Seven executive-compensation experts told Reuters that share liquidations by Moderna executives show the incentives biotech executives have to highlight development milestones, even for drugs that often don’t get approved or sold. Such optimistic statements could cause investors to overpay for company shares or create false hope concerning a possible coronavirus vaccine. “This may be their one shot at making a boatload of money if the vaccine doesn’t work out,” said Jesse Fried, a Harvard Law School professor who wrote a book about executive compensation, adding that Moderna’s chiefs have a powerful motivation to “keep the stock price up.”
Reuters has not found evidence that Bancel, Zaks or Moderna have exaggerated the company’s vaccine progress.
Uncertainty remains concerning how effective a vaccine will be in fighting the coronavirus, as it is still unclear how long one can remain immune to the virus.
Moderna launched a 30,000-subject trial of a COVID-19 vaccine that could clear the way for regulatory approval and widespread use by the end of this year, the company said last week.
The trial is one of the first late-stage studies supported by the Trump administration’s effort to speed development of measures against the novel coronavirus, adding to hope that an effective vaccine will help end the pandemic.
- 07/31/2020 – have a clear mind about this. Not as simple as we expect
The Three Key Hurdles for a Coronavirus Vaccine to Clear
Making a safe, effective Covid-19 vaccine will be hard enough, but distributing it and building trust for widespread participation will be even harder
- First, will it work? Still, we have ample reason to be guardedly optimistic that some vaccines will provide some level of protection, and that this will be demonstrated before the end of 2020. Some vaccine candidates may be duds, and others’ efficacy may wane within just a few months—something that will, of course, take many months to know. Different vaccines may be more or less effective, and some vaccines may work less well for some groups. And most vaccines never make it to approval; many of today’s promising candidates may fail. We simply don’t know. That’s what studies are for.
- Second, will it be safe?
- Third, can we get it to people?
- 07/31/2020 – Negotiation on stimulus plan is still going on. Talks between top Democrats and White House officials were scheduled for Saturday morning, continuing efforts to reach an accord before the Senate begins a scheduled August recess at the end of next week.
Jobless Aid Expires as Talks Continue on Coronavirus Package
Democrats and Republicans remain at odds on level of unemployment payments, state and local aid
- 07/31/2020 – Merck’s progress on therapeutic: has scheduled “very large pivotal studies” for its oral coronavirus treatment in collaboration with Ridgeback Biotherapeutics as early as September
Merck aims to start ‘large pivotal’ studies on coronavirus treatment in September
- Merck has scheduled “very large pivotal studies” for its oral coronavirus treatment in collaboration with Ridgeback Biotherapeutics as early as September, a company executive said Friday.
- The experimental oral therapeutic, known as MK-4482, that would fight against Covid-19 is currently in phase two trials.
- The goal of future studies will be to prove the drug can reduce the duration of Covid-19 symptoms and, more importantly, keep people from developing serious illness that could send them to the hospital.
- 07/30/2020 – J&J to start phase 3 trail in the mid of Sept
Johnson & Johnson starts human study of COVID-19 vaccine after promising monkey data
(Reuters) – Johnson & Johnson on Thursday kicked off U.S. human safety trials for its COVID-19 vaccine after releasing details of a study in monkeys that showed its best-performing vaccine candidate offered strong protection in a single dose.
When exposed to the virus, six out of six animals who got the vaccine candidate were completely protected from lung disease and five out of six were protected from infection as measured by the presence of virus in nasal swabs, according to the study published in the journal Nature.
“This gives us confidence that we can test a single-shot vaccine in this epidemic and learn whether it has a protective effect in humans,” Dr. Paul Stoffels, J&J’s chief scientific officer, told Reuters in a telephone interview.
The drugmaker said it had started early-stage human trials in the United States and Belgium and would test its vaccine candidate in more than 1,000 healthy adults aged 18 to 55 years, as well as adults aged 65 years and older.
The U.S. government is backing J&J’s vaccine effort with $456 million in funding as part of a spending spree aimed at speeding production of a vaccine to end the pandemic, which has infected millions and killed more than 660,000 people.
Stoffels said prior tests of this type of vaccine in other diseases found that a second shot significantly increases protection. But in a pandemic a single-shot vaccine has a significant advantage, sidestepping a lot of the logistical issues involved in getting people to come back for their second dose.
The company plans to take up the question of one or two doses in its phase 1 trial.
Depending on those results, J&J plans to start large-scale, phase 3 testing with a single-shot regimen in the second half of September. Around the same time, the company will start a parallel phase 3 study testing a two-shot regimen of the vaccine, Stoffels said.
“The Johnson & Johnson vaccine is exciting because it’s a single dose,” White House Coronavirus Task Force Coordinator Deborah Birx said in an interview with Fox News.
Having one dose “show protection in monkeys like the other vaccines have shown with two doses does shorten the time period for development because your readout becomes 30 days quicker,” she said.
J&J’s vaccine uses a common cold virus known as adenovirus type 26, or Ad26, to ferry coronavirus proteins into cells in the body, causing the body to mount an immune defense against the virus.
In the monkey study, scientists from J&J and Harvard’s Beth Israel Deaconess Medical Center studied seven different potential vaccines in 32 animals and compared the results to 20 control animals who got placebo shots.
Six weeks later, all of the animals were exposed to the SARS-CoV-2 virus. All 20 animals that received the placebo developed high levels of virus in their lungs and nasal swabs.
In the best-performing candidate, which J&J selected for human testing, none of the animals had virus in their lungs and only one showed low levels of virus in nasal swabs. Lab tests showed they all had developed antibodies capable of neutralizing the virus after a single shot.
- 07/28/2020 – Pfizer starts phase 3 trial on Monday 07/27/2020, encouraging news
Pfizer Beats Forecasts as Vaccine Trial Enters Final Stage
Drugmaker on Monday started pivotal phase of testing Covid-19 vaccine in 30,000 people
The company’s candidate vaccine to protect against Covid-19, which it is developing with BioNTech, began Phase 3 testing this week. Last week, the U.S. agreed to pay the companies $1.95 billion for 100 million doses of the vaccine. Moderna Inc. and Johnson & Johnson are among the other American companies racing to roll out a successful vaccine for the disease, which continues to spread in the U.S.
- 07/27/2020 – vaccine phase-3 trials: Moderna today, Pfizer in July, AstraZeneca August, J&J Sept.
Moderna, Pfizer Coronavirus Vaccines Begin Final-Stage Testing
Drugmakers to enroll 30,000 subjects across U.S., in separate trials to determine whether their vaccines prevent symptomatic Covid-19
Pfizer, BioNTech nab fast track tag, prep for major phase 3 COVID-19 vax test this month
Pfizer and German mRNA partner BioNTech have grabbed an FDA fast track label as they look to start a late-stage, 30,000-patient COVID-19 vaccine test before the month is out.
Coronavirus Vaccine Candidates’ Pivotal U.S. Testing to Start This Summer
The last stage of testing for Moderna’s vaccine would begin in July, an NIH official says, followed by candidates from AstraZeneca and J&J
Pfizer’s trial, which will begin in the U.S. but expand overseas to include about 120 sites, will evaluate a vaccine developed with partner BioNTech SE. BNTX 2.80% The shot is one of four candidates the companies evaluated.
Moderna Inc.’s MRNA 9.15% vaccine is set to be first, starting in July, followed in August by one co-developed by Oxford University and AstraZeneca AZN 1.40% PLC and in September by Johnson & Johnson’s JNJ -0.63% , he said.
- 07/27/2020 – Moderna COVID-19 vaccine could be ready for use by end of year, and a readout from the trial could come by November, December, or even earlier. Scientists could know whether a potential coronavirus vaccine by Moderna works as early as October, but will likely have the full results by November, Fauci said.
Moderna COVID-19 vaccine could be ready for use by end of year, U.S. says
Moderna Inc’s (MRNA.O) vaccine against COVID-19 could be rolled out by the end of this year, U.S. officials said on Monday, after the drugmaker announced the start of a 30,000-subject trial to demonstrate it is safe and effective, the final hurdle prior to approval by global regulators.
Anthony Fauci, the top U.S. infectious disease official, said a readout from the trial could come by November, December, or even earlier. Fauci said he was “not particularly concerned” about the vaccine’s safety after seeing data from earlier, smaller trials.
The study also seeks to determine whether the vaccine can prevent coronavirus-related deaths.
Trial volunteers will receive two injections about 28 days apart of either 100 micrograms of mRNA-1273 or a placebo.
Results of a small early-stage study published earlier this month showed volunteers who got two doses of Moderna’s vaccine had levels of virus-killing antibodies that exceeded the average seen in people who had recovered from COVID-19.
Moderna said it remains on track to deliver about 500 million doses a year, and possibly up to 1 billion doses a year, beginning 2021.
Dr. Anthony Fauci isn’t ‘particularly concerned’ about safety of Moderna coronavirus vaccine
“It’s a novel technology. We are certainly aware of the fact that that there’s not as much experience with this type of platform as there are with other standards,” Fauci, director of the National Institute of Allergy and Infectious Diseases, told reporters on a conference call alongside National Institutes of Health Director Dr. Francis Collins. “I’m not particularly concerned. But I don’t want a lack of severe concern get in the way that we are keeping an open mind to look for any possible deleterious effects as we get into and through the phase three trial.”
Scientists could know whether a potential coronavirus vaccine by Moderna works as early as October, but will likely have the full results by November, Fauci said.
- 07/26/2020 – encouraging news from Moderna: more funding coming, and Phase 3 study on July 27
Moderna gets further $472 million U.S. award for coronavirus vaccine development
(Reuters) – Moderna Inc said on Sunday it has received an additional $472 million from the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA) to support development of its novel coronavirus vaccine.
A Phase 3 study, conducted in collaboration with the National Institute of Allergy and Infectious Diseases, will begin on July 27 and involve about 30,000 participants, according to the company.
Moderna said it remains on track to be able to deliver about 500 million doses per year, and possibly up to 1 billion doses per year, beginning in 2021.
- 07/26/2020 – Kudlow and Mnuchin still think economy is a V shape recovery. Mnuchin predicts that 3rd quarter will be a great one. We will wait and see. If it is not, no chance for Trump to get reelected.
Trump Advisers Still Touting ‘V-Shaped’ Economic Recovery
Kudlow points to improving data on home sales and manufacturing, predicts unemployment will decline despite surges in new coronavirus infections
“We always said the second quarter was going to be a very bad quarter, again that’s not for economic reasons, that’s for health reasons,” Mr. Mnuchin said Sunday.
“So we do think you’re going to see a very big rebound,” Mr. Mnuchin said, citing a consensus estimate for 17% economic growth at an annual rate in the third quarter.
- 07/23/2020 – Mitch McConnell said new stimulus plan will come by end of July. The bill will be introduced next week and WH requested additional time to review it.
CARES Act 2 framework finalized: What will be included
Bill combines policies that worked well in CARES Act with new proposals
The bill represents “an agreement in principle” with the Trump administration, McConnell noted, and will be introduced next week.
The White House has, however, requested additional time to review the legislation.
Senate Majority Leader Mitch McConnell on Thursday outlined some of the measures that Republicans have included in the framework for a proposed “CARES 2” bill, which targets support for kids, jobs and health care.
“We will propose to continue and renew some of the most successful CARES Act policies, while adding bold, new ideas,” McConnell said on the Senate floor Thursday.
In a Twitter post, McConnell said the framework is “tailored to this phase of the crisis to deliver more relief to the American people.”
- 07/23/2020 – weekly unemployment claims arise is not good sign
Rise in Weekly Unemployment Claims Points to Faltering Jobs Recovery
Initial claims climb for first time in nearly four months to 1.4 million amid uptick in coronavirus cases
Filings for weekly unemployment benefits rose for the first time in nearly four months as some states rolled back reopenings because of the coronavirus pandemic, a sign the jobs recovery could be faltering.
Initial unemployment claims rose by a seasonally adjusted 109,000 to 1.4 million for the week ended July 18, the Labor Department said Thursday, halting what had been a steady descent from a peak of 6.9 million in late March, when the pandemic and business closures shut down parts of the U.S. economy.
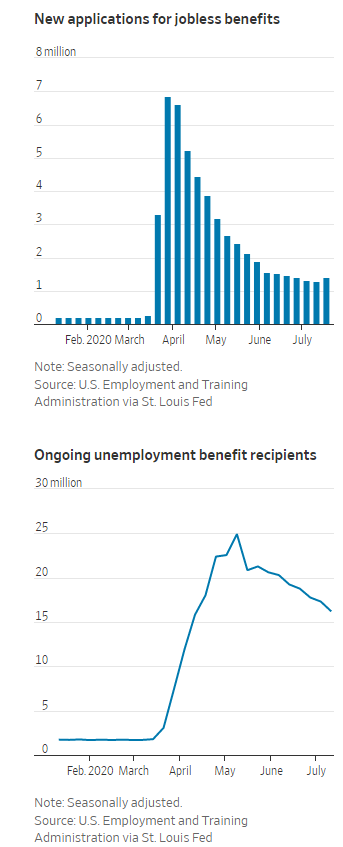
- 07/23/2020 – promising news from China on vaccine
China Says It Will Have Covid-19 Vaccine Ready This Year
Chinese companies make headway in global race to produce the first coronavirus vaccine
- 07/23/2020 – compromise on payroll tax cut will help solidify the stimulus plan
Mnuchin: Payroll Tax Cut Won’t Be in Senate Republican Coronavirus Stimulus Bill
Measure could be in future relief legislation, Treasury secretary says
WASHINGTON—Senate Republicans won’t include a payroll tax cut, a measure long sought by President Trump, in the coronavirus relief legislation they are planning to release publicly on Thursday amid opposition from lawmakers of both parties.
“It won’t be in the base bill,” Treasury Secretary Steven Mnuchin said during a Thursday interview with CNBC, adding that the measure could be included in future coronavirus relief bills.
In their bill, Republicans are expected to include an extension of enhanced jobless benefits, but at a lower level than the continuation of the current $600 a week that Democrats are seeking.
“We want to make sure that the people who are out there that can’t find jobs do get a reasonable wage replacement. So it will be based on approximately 70% wage replacement,” Mr. Mnuchin said.
The release of the GOP plan will begin a new leg in the race to pass a fifth relief package before the current federal unemployment benefits expire on July 31. While Republicans face internal divisions on a range of issues, including whether to even pursue a new bill, Democrats have largely rallied around a $3.5 trillion bill passed in the House in May.
- 07/22/2020 – government contract for vaccine is promising news
Trump applauds government contract with Pfizer and BioNTech, says he thinks vaccine is ‘a winner’
President Donald Trump called the federal government’s $1.95 billion deal with Pfizer and biotech firm BioNTech “a historic agreement” that will help the country distribute a coronavirus vaccine in record-breaking time.
“Hopefully the approval process will go very quickly, and we think we have a winner there. We also think we have other companies right behind that are doing very well on the vaccines, long ahead of schedule,” Trump told reporters at the White House on Wednesday.
- 07/20/2020 – starting the negotiation on the next stimulus bill, result uncertain
Mnuchin says leaders are ‘starting with another trillion dollars’ for next coronavirus stimulus bill
Mnuchin said another focus is developing a vaccine for COVID-19
Treasury Secretary Steven Mnuchin provided some details regarding the federal government’s next coronavirus stimulus package, noting that the starting point for how much money will go into it is $1 trillion dollars.
Speaking alongside President Trump, Vice President Mike Pence, Senate Majority Leader Mitch McConnell, R-Ky., and House Minority Leader Rep. Kevin McCarthy, R-Calif., in the Oval Office, Mnuchin said that “the focus is kids and jobs,” and that the goal is to have it in place by the end of this month.
“We’re focused on starting with another trillion dollars, we think that will make a big impact,” Mnuchin said.
- 07/20/2020 – promising news on vaccine and drug treatment for covid-19
Trials of a vaccine and new drug raise hope of beating covid-19
The latest tests with Oxford University’s vaccine, and interferon beta, look promising
The Oxford vaccine is furthest ahead with such efficacy trials. A 10,000-patient trial in Britain has almost finished recruiting, and recruitment is rising quickly for a trial in Brazil. Trials in South Africa have just started, and another will begin in America in the coming weeks. If everything goes well it could be clear to researchers by the end of August whether or not the vaccine works. When the results of the first of these trials are available, assuming they are positive, regulators will have to decide whether there are enough data to allow for some sort of emergency approval before further trials are conducted. That could happen as early as October.
The news from Oxford came on the same day as the announcement of a possible drug treatment for covid-19. Synairgen, a small British biotechnology company, said it will soon present findings that its inhaled form of a substance called interferon beta is effective. When this drug was given to those with the illness in a trial involving 100 patients (small, by the standards of these things), it significantly reduced the numbers who then went on into intensive care. The odds of requiring ventilation were reduced by 79% and patients were two to three times more likely to recover.
Outsiders have urged caution in interpreting these results, because Synairgen has not yet released information about how it conducted its trial or what sort of patients were recruited into it. Nor have the findings been peer-reviewed. However, if the claims made are confirmed, this work would represent an important advance in the ability to treat covid-19. Taken together, then, these two announcements add to hope that science will provide an exit strategy for covid-19 some time this year.
- 07/20/2020 – promise trials results from AstraZeneca, Pfizer-BioNTech and CanSino
Coronavirus Vaccine Data Raises Hope for Trio of Candidates
The results buoyed the prospects a successful vaccine could arrive before year-end
The prospects of successfully developing a coronavirus vaccine as soon as this year were buoyed Monday when three of the world’s leading candidates reported positive early trial data.
Vaccines being developed by University of Oxford researchers and AstraZeneca AZN -3.66% PLC; Pfizer PFE +0.91% Inc. and German partner BioNTech BNTX +2.84% SE; and China’s CanSino Biologics 6185 2.17% all reported fresh updates showing their shots generated immune responses and were safe to use.
Oxford University-AstraZeneca Covid-19 Vaccine Front-Runner Shows Promise in Trials
Early results put researchers on track for a shot that could be ready for mass production as soon as September
LONDON—A leading coronavirus-vaccine candidate being developed by University of Oxford researchers and AstraZeneca AZN -3.36% PLC showed positive results in early trials, according to results published Monday, raising the prospect that a shot could be available by the end of this year.
A study of 1,077 healthy adults who received the vaccine showed it produced two kinds of immune response that could defend a body against Covid-19, according to results published in the Lancet, a British medical journal. The vaccine caused no serious side effects, the study found.
Pfizer-BioNTech potential coronavirus vaccine shows promise in second early trial
German biotech BioNTech and U.S. drugmaker Pfizer Inc on Monday said data from an early-stage trial of their experimental coronavirus vaccine showed that it prompted an immune response and was well-tolerated, similar to results seen in prior early test.
- 07/18/2020 – uncertainty on when and whether the next stimulus plan will come
Depleted Trump Economic Team Faces Major Test Over Extending Coronavirus Relief Efforts
Next aid package will be critical in shaping the pace of recovery from the biggest crisis since the Great Depression
- 07/18/2020 – cautiously optimistic on Moderna’s latest vaccine trials
Video: An infectious disease expert explains the results from Moderna’s latest vaccine trials
Biotech company Moderna, one of many organizations developing a vaccine for COVID-19, published results from an early-stage test of its experimental mRNA vaccine in the New England Journal of Medicine July 14. Vanderbilt University Medical Center staff scientist and protein chemistry expert Sanjay Mishra explains what the results of the phase 1 trial mean.
Can you explain what the company’s mRNA vaccine is and why it is different?
Vaccines are meant to train the immune system to attack the disease-causing virus. In the case of SARS-CoV-2, there is a spike protein, or the S-protein, which is the flag that the immune system needs to recognize as the signature of the virus. So the goal of a vaccine is to train your immune system to recognize the S-protein, and then trigger the immune response. This S-protein is the standard in all coronaviruses, that’s why they’re called coronaviruses, because the “corona” is the crown.
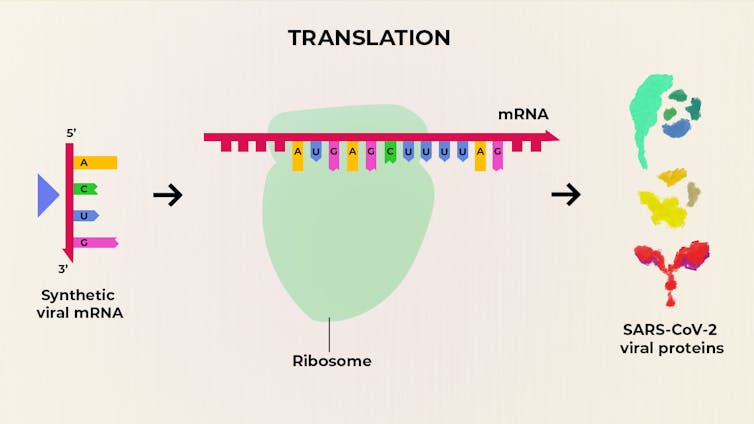
Traditionally, the vaccines involved either a weakened virus, or a preparation of the virus that would have contained (in this case) the spike protein. In the cleanest method you would have produced the spike protein in the lab and then you would have used that as the immunization candidate.
All those methods are time-consuming and require extensive quality control. And usually there is a lot of headache in scaling up from lab to production. Moderna’s vaccine and another candidate vaccine bypasses this process by using mRNA , or messenger RNA. It is genetic coding material which will help your body produce that protein. This way you don’t have to deal with the production of the protein in the lab and risk creating an impure protein sample, which can be clinically difficult to standardize and then can be dangerous as well.
So in this case, what you’re giving is not the protein or part of the virus, but a synthetic messenger RNA in a lipid droplet.
How did you feel when you heard the news?
I feel cautiously optimistic. The study provides promising data on the safety and immunogenicity, or the ability to provoke an immune response. It is a good starting point for training the immunity of the body. But if I can paraphrase Robert Frost, we still have miles to go before we sleep.
Vaccine development is complex and there’s a lot more work that needs to be done before this can become an actual marketable candidate.
This first batch of data is from the 18- to 55-year-old group. We do not know what the dosing would be for the older age group, which is the most vulnerable to COVID-19. As we age, we do not produce as many antibodies, which generally leads to poor vaccine response. So the question is: Will they have to go for a higher dose, which is usually the case in flu vaccines. The higher dosage, which is 250 micrograms, has led to somewhat more severe side effects in this study. So then how would that be balanced? It is still difficult to say.
What other vaccines are being developed?
There are 178 COVID-19 vaccines in various stages of development and 14 are leading to human trials, including from AstraZeneca and others. There are more potential candidates from Merck, Johnson & Johnson and others. There is a similar vaccine that is already being tested by Pfizer and BioNTech, and that has also shown positive results at the lower doses.
- 07/18/2020 – Even the master of caution on vaccines, Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, thinks a signal of vaccine efficacy might arrive in September. If data from trials emerges to suggest that a vaccine works, the Food and Drug Administration (fda) will have to decide whether to grant an Emergency Use Authorisation (eua).
Operation Warp Speed, Donald Trump is hoping for a covid-19 treatment by November
He might get one
The government’s money is not universally appealing to the pharma industry. The giant drug firm Pfizer has rejected cash from ows. Its boss, Albert Bourla, says working with the government would slow the firm down. That fear seems to be justified. Work by Moderna, a biotech firm, appears to have been delayed amid reports of squabbles between the firm and the federal government over the design of trials.
Yet with the eagerness of the pharma sector to find treatments, along with the broad range of investments made by ows (as well as other governments) there has been a lot of progress in the search for tests, drugs and vaccines. AstraZeneca has started late-stage trials, and Moderna and Pfizer are expected to do the same before the end of the summer. Even the master of caution on vaccines, Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, thinks a signal of vaccine efficacy might arrive in September. If data from trials emerges to suggest that a vaccine works, the Food and Drug Administration (fda) will have to decide whether to grant an Emergency Use Authorisation (eua).
Assuming these vaccines work, a best-case scenario could put an eua for a vaccine well before the November election. A signal from the trials of the Regeneron drug is expected by the end of the summer. If it works, the federal government looks likely to be sitting on most of the world’s supply of the drug. All this means that Mr Trump has a reasonable chance at being right about there being a drug or vaccine by the end of the year. More significantly, analysts at Morgan Stanley think that the sort of early data that now looks possible in the coming months, would allow investors to “look through” any negative headlines in the economic recovery. This has the potential to help Mr Trump at the polls.
Successful therapies, particularly those supported by ows, would give Mr Trump something to brag about on the campaign Zoom trail. Yet it seems unlikely to blunt much of the effect of the disastrous increase in cases of covid-19 (see article). The administration is keen to paint ows in heroic terms, describing it as the “one of the greatest scientific and humanitarian accomplishments in history.” The reality is that even if international efforts help to create the knowledge to defeat covid-19, America looks unwilling to share the humanitarian gains outside its own borders.
The administration has shunned international efforts to co-operate on vaccines. On July 7th it said it would begin the process of withdrawing from the World Health Organisation, which plays a key role in organising the global distribution of vaccines, drugs and diagnostics. There is a strong argument that vaccines will be more efficiently deployed when delivered to the high-risk populations around the world rather than through near universal vaccination of a few rich countries such as America. That argument has not been very persuasive with this administration, but Mr Trump did promise America First.
- 07/17/2020 – roll back of regulation is good for economy
Trump rolls back environmental protections to accelerate infrastructure projects
Trump overhauling the National Environmental Policy Act
Trump has made cutting regulations and getting rid of red tape a hallmark of his presidency.
“The last administration increased the Federal Register by 16,000 pages of job-killing regulations. Under my administration, we have cut the Federal Register by nearly 25,000 pages. More than any president in history,” Trump said Wednesday.
A New York Times analysis counts 67 environmental rules and regulations that have been reversed by the Trump administration. 33 more environmental regulations are in the process of being reversed.
- 07/16/2020 – A bill that could be voted on as soon as next Friday
Mnuchin, Kudlow in epic clash over next stimulus package A White House divided: Economic officials at odds on next phase of stimulus
A civil war is taking place inside the White House pitting Treasury Secretary Steven Mnuchin against National Economic Council chief Larry Kudlow, one of the nation’s foremost supply-side economists, over a key provision of the fourth round of stimulus amid COVID-19 lockdowns, FOX Business has learned.
Mnuchin has blocked out next week to meet with lawmakers and hammer out a bill that could be voted on as soon as next Friday, people with knowledge of the matter tell FOX Business.
- 07/16/2020 – Democrats want more than 1.3T on next stimulus plan
Nancy Pelosi says coronavirus aid bill will cost at least $1.3 trillion, but ‘that’s not enough’
- House Speaker Nancy Pelosi says a developing coronavirus relief bill will cost at least $1.3 trillion, but argues it is not enough as the pandemic spreads unabated.
- Congress has only days to extend a $600 per week enhanced unemployment benefit that has been a critical lifeline for jobless Americans during the economic crisis.
- Pelosi and Senate Majority Leader Mitch McConnell need to resolve several key issues, including how to help schools reopen safely and whether to send another round of direct payments, provide more relief to state and local governments and provide assistance to renters and homeowners.
- 07/15/2020 – vaccine, stimulus plan and infrastructure plan might can boost the market further, especially the depressed sector, like finance, small business and construction.
Stocks jump on coronavirus vaccine hopes, Trump’s infrastructure plan
Goldman Sachs saw record-breaking performance from its investment bank
- 07/15/2020 – China’s growth might be the near future of US’s growth
China’s economy grows 3.2%, first economy to rise since coronavirus pandemic
The expansion was a dramatic improvement over the previous quarter’s 6.8 percent contraction
- 07/15/2020 – some details of the upcoming stimulus bill. The stimulus package might be introduce next week. I need to get ready for it.
Next Stimulus Bill Will Likely Have These Three Major Changes
Senate Majority Leader Mitch McConnell (R-KY) stated in a July 13th interview that he and Treasury Secretary Mnuchin have “been working on this [the next stimulus bill] for several weeks.” Discussion points include another stimulus check, extending unemployment benefits, and even funding to help schools reopen.
McConnell is also disclosing several significant changes that Republicans and Democrats will discuss to start the legislative process but may cause some friction.
The “menu of options” is continually changing as the national health and employment situation changes. For instance, the stimulus a month ago was more likely to have pro-growth incentives like “back to work” bonuses as states were reopening rapidly and infection rates were low.
Fast forward to today and the near-term economic outlook is more uncertain. Multiple states are ratcheting back their reopening plans to counter a surge in new COVID-19 cases. Big bank CEOs in their July 2020 earnings calls mention they are preparing for a wave of personal finance malaise if federal stimulus benefits cease.
When the House of Representatives passed the Heroes Act, the stimulus measures weres very similar to the Cares Act. It seems like the next stimulus bill will like have have a few major differences.
Cares Act
Let’s take a look at what was in the Cares Act that became law in late March.
Some of the signature pieces of this bill include:
- One-time $1,200 stimulus check for adults (and $500 for children)
- Weekly $600 federal unemployment benefit until July 31st
- Waived interest payments on most federal student loans until September 30th
- “Paycheck Protection Program” (PPP) small business loans
- No 10% early withdrawal penalty from retirement accounts for qualifying coronavirus-related expenses
- Temporary waiver for required minimum distributions (RMDs)
There are many positives in this bill including stimulus checks for those who do not file tax returns and unemployment benefits for those who don’t qualify for state unemployment insurance.
However, there are several glaring flaws as the Cares Act legislative process was rushed. The general public and the government agencies responsible for administering the benefits had to work out several problems to get the aid money moving.
One of the most immediate frustrations was determining who qualifies for a stimulus check. Income phaseouts began at $75,000 for single taxpayers and $150,000 for joint taxpayers based on your most recent tax return info. It turns out several groups could not receive full payment including college students and spouses without a Social Security Number.
Also, states were not ready to issue the $600 weekly federal benefit to the self-employed and those who don’t qualify for state unemployment insurance. States had to create a new application portal and then wait for federal funding to arrive.
Heroes Act
Even as the ink was drying on the Cares Act, lawmakers began working on various proposals for a follow-up stimulus. The only plan to pass a full chamber is the Democrats’ Heroes Act.
The act passed in the House in May 2020 but is a non-starter in the Republican-led Senate.
One reason why the Heroes Act most likely won’t be the next stimulus bill is because of its $3 trillion price tag – which is itself even higher than the $2.2 trillion CARES Act. Senator McConnell wants the next stimulus bill to be around $1 trillion—which means less aid all around.
The next stimulus bill will likely be smaller in size than the Heroes Act and Cares Act. But Congress may use the best parts from both proposals to reach a bipartisan agreement.
Democrats may push for these key Heroes Act proposals in this summer’s bill:
- Extend weekly federal unemployment benefits until early 2021
- $1,200 stimulus payment for adults and children (up to three children)
- Pandemic Premium Pay for essential workers
- A temporary moratorium on evictions for up to 12 months
The Heroes Act also addresses some of the Cares Act benefit errors. Specifically, helping more people qualify for a stimulus check. For instance, spouses and children who only have a taxpayer identification number (TIN) can receive payment too.
There are several overlapping benefits between the Republican and Democrat proposals. But each party has different positions on the total cost and benefit scope.
The bipartisan compromise may offer a second stimulus check and extend federal unemployment insurance to win Democratic support. Still, fewer people will qualify to keep total cost low and honor Republican wishes for fiscal prudence.
What Are The Three Big Changes We May See?
Here are the latest insights we have about what Congress may propose next week. Below are some of the benefits that lawmakers proposed in the Cares Act and Heroes Act that we may see again in this bill.
1. Lower Federal Unemployment Benefits
Extending the weekly $600 federal unemployment benefits beyond the current July 31st sunset date is probably the largest surprise—so far.
Until a few weeks ago, the White House and congressional Republicans adamantly opposed lengthening the $600 weekly benefit. Then the number of new coronavirus cases began increasing and some businesses had to start shutting down again in various states.
While a benefits extension seems inevitable, the revised federal unemployment insurance will not be as generous for most recipients. Treasury Secretary Mnuchin hinted in early July the new benefits may cap at the worker’s regular weekly income.
Lawmakers must decide if they will adopt the Heroes Act proposal of extending the benefits until January 31, 2021. The new Congress elected in November would discuss the next stimulus bill.
The stricter benefit caps stem from a research paper by the National Bureau of Economic Research. Their study finds that approximately two-thirds of laid-off workers earn more collecting unemployment than their standard wage. This same paper also suggests that 20% of recipients may earn double their usual weekly paycheck.
While the $600 weekly benefit won’t make you wealthy, Republicans routinely suggest the enhance benefits create a disincentive to work in states with a low cost of living.
For example, the maximum weekly unemployment benefit in Tennessee is $275 plus the $600 the Cares Act benefit. Earning both benefits is equivalent to an annual pre-tax salary of $45,500—a comfortable income in many parts of Tennessee.
Another reason Republicans may rally behind extending unemployment benefits is if businesses get coronavirus liability protection. Senator McConnell wants the liability shield to apply from December 2019 through 2024 to minimize potential lawsuits and business closures.
2. Smaller and More Targeted Stimulus Checks
A second stimulus check is another idea that Republicans have waffled over since the Cares Act passage. It now feels almost certain that the IRS will distribute a second check, but for how many and for how much?
Like the federal unemployment benefits, it’s likely that fewer people will get a second stimulus payment.
McConnell has said that he wants to target them to those most hurt by the pandemic – and he’s mentioned $40,000 in income as a possible income limit. This leads us to believe that earning an annual income of $40,000 or less will most likely qualify. The most-affected households earn an income below this threshold. This new income limit is lower than the $75,000 limit the Cares Act and Heroes Act share.
We don’t know how large the second stimulus check will be yet for adults or students. The payment will likely be at least $1,200 for adults and potentially higher. President Trump is on record on July 1st interview saying: “I support actually larger numbers than the Democrats, but it’s got to be done properly… I want the money getting to people to be larger so they can spend it.”
The payment amount can be higher than the first check if you qualify.
This check should arrive sooner than the Cares Act payment as the IRS should have your direct deposit details on file. Similar to the first check, this second payment will be an “advance” refundable tax credit on your 2020 federal tax return.
3. Payroll Tax Cut
A payroll tax waiver on Social Security and Medicare tax withholdings is the one stimulus measure with President Trump’s constant support.
The current tax rate is 6.2% for Social Security (on the first $137,700 in annual income) and 1.45% for Medicare. This waiver would temporarily reduce the tax amount and increase your take-home pay.
This benefit helps those who don’t receive the second stimulus payment or unemployment insurance. However, this waiver doesn’t help the unemployed and retirees.
Legislators will need to decide on how much to reduce the payroll tax. President Obama enacted a similar tax holiday during the Great Recession to lower the rate from 6.2% to 4.2% in 2011 and 2012.
Another potential contention point is a waiver increases the existing funding shortfalls for Social Security and Medicare. Reducing the rate for those who keep working can result in a future tax hike to offset the lost revenue from 2020.
When Will the Next Stimulus Check Bill Pass?
The Senate and House of Representatives have a narrow working window to reach a bipartisan agreement. Both chambers have a scheduled multi-week August recess. Not passing a bill in July or August means Congress might pause stimulus talks until after Labor Day weekend in September.
House Speaker Nancy Pelosi states she is willing to delay or cancel the House recess to pass the next stimulus check bill.
Passing the bill this summer minimizes any potential coverage gap for federal unemployment benefits. Quick passage also means people can receive the second stimulus check as early as September.
- 07/14/2020 – Moderna’s vaccine phase 3 is coming on July 27
Moderna will begin late-stage coronavirus vaccine trial on July 27
- Moderna will begin its late-stage trial testing a potential vaccine to prevent Covid-19 on July 27, according to a posting published Tuesday on ClinicalTrials.gov.
- The trial will enroll 30,000 participants across 87 locations, according to the website.
Dr. Anthony Fauci, the nation’s top infectious disease expert, has often touted Moderna’s potential vaccine.
On Monday, he said he’s “cautiously optimistic” scientists will be able to create at least one safe and effective vaccine by the end of the year or early 2021.
- 07/14/2020 – next stimulus plan is coming?
House Speaker Nancy Pelosi says she would delay August recess to pass coronavirus relief bill
- House Speaker Nancy Pelosi said she would delay or cancel her chamber’s August recess to pass a coronavirus relief bill.
- Congress looks to approve additional aid legislation before the end of the month as a $600 per week federal unemployment benefit is set to expire.
- Democrats and Republicans have to resolve disagreements over issues including jobless benefits, state and local government relief, direct payments to Americans, and liability protections for doctors and businesses.
- 07/13/2020 – talk on next phase of stinulus plan
McConnell in talks with Mnuchin on next phase of coronavirus relief
Senate Majority Leader Mitch McConnell (R-Ky.) said Monday that he is in discussions with Treasury Secretary Steven Mnuchin on the next phase of coronavirus relief and predicted the Senate will begin working on the legislation more widely next week.
McConnell said he’s been working closely with Mnuchin over the last few weeks and will begin sharing the emerging work product with the rest of the Senate GOP conference after colleagues return to Washington on July 20.
“I’m predicting we will have one more rescue package, which we’ll begin to debate and discuss next week,” McConnell said during an appearance at Baptist Health Hospital in Corbin, Ky.
- 07/13/2020 – conflict of US and China on HK
U.S. weighs limited options to deal with China over Hong Kong: WSJ
- 07/12/2020 – watch out the upcoming earnings
Wall Street’s Earnings Forecast: Cloudy With a Chance of Turbulence
Coronavirus uncertainties prompted hundreds of companies to pull their guidance, blinding analysts and investors
- 07/12/2020 – be fully aware of the implication of vaccine development
As Covid-19 Vaccine Development Pushes Ahead, Researchers Probe Safety
Among potential concerns is rare complication that could worsen coronavirus’s severity
New Coronavirus Cases in U.S. Top 60,000 for Third Straight Day
California, Florida, Texas, Arizona and Georgia report near-record daily cases
- 07/09/2020 – 2Q earning disaster is coming
‘Disaster’ U.S. earnings loom, but investors try and look beyond
- 07/09/2020 – another CV-19 relief bill might come before the end of July. Watch out how do two parties compromise or not compromise on the bill
Treasury’s Mnuchin backs narrower coronavirus aid package as talks with Congress resume
- Treasury Secretary Steven Mnuchin said Congress and the White House will aim to craft another coronavirus relief bill before the end of the month.
- He said the White House backs direct payments to individuals but did not specify what kind of income cap the administration would support.
- Mnuchin added that the administration wants to change the enhanced federal unemployment benefit set to expire at the end of July, but did not say how it wants to structure the proposal.
- His stance puts him at odds with House Democrats, who want a much more sweeping aid package as the pandemic continues to ravage the country.
Democrats put nearly $1 trillion in aid for state and local governments in a $3 trillion relief bill the House passed in May. House Speaker Nancy Pelosi, D-Calif., considers the funds a priority.
McConnell has repeatedly said he wants to see liability protections for doctors and businesses in any new relief legislation.
- 07/07/2020 – the conflict of US and China is accelerating, this might add market volatility
- 07/07/2020 – New stimulus plan might come in July and August
BREAKING: Rise in #COVID cases prompt
to renew push to get Senate GOP to compromise w Dems and reach a deal on a new stimulus in July. Trump worried covid cases could jeopardize nascent recovery as election approaches. We discuss whats on the table NOW
Hassett Sees Another Stimulus Bill From Congress Before August Recess
White House adviser says the odds of a ‘Phase Four’ stimulus package ‘are very, very high’
WASHINGTON—A top economic adviser to President Trump said the White House “would definitely support” another round of aid to shore up the economy as U.S. businesses begin to reopen amid the coronavirus pandemic.
White House economic adviser Kevin Hassett said Tuesday the odds of a “Phase Four” stimulus package “are very, very high,” even if data on output and jobs continue to surpass expectations. On Friday, the Labor Department reported that U.S. employers added 2.5 million jobs in May and that the unemployment rate, which economists had expected to soar to 19.5%, instead fell to 13.3%.
McConnell eyes next coronavirus package after July recess
Negotiations are likely to be even more painful in this round of talks.
Senate Majority Leader Mitch McConnell on Tuesday gave his clearest signal yet that Republicans are willing to move swiftly on another coronavirus relief package, after some states have seen a spike in cases.
The Kentucky Republican said that the Senate will focus on the next coronavirus package when it returns from the two-week July 4 recess, with the goal of finishing before both chambers depart for their lengthy August break.
Next stimulus package will be jobs-focused: Mnuchin
Mnuchin says ‘bunch’ of different ideas are being discussed
- 07/06/2020 – CV-19 is here to stay until vaccine comes?
Covid-19 is here to stay. People will have to adapt
The world is not experiencing a second wave: it never got over the first
- 07/01/2020 – when masks are on, infection rates might drop. Good for country
Trump says ‘masks are good,’ but doubts national mandate is needed as coronavirus cases rise
- President Donald Trump said that he’s “all for masks,” but doesn’t think the U.S. needs a national mandate for people to wear them during the coronavirus pandemic.
- “I don’t know if you need mandatory,” Trump said when asked in a Fox Business Network interview whether he would support a national order for people to wear masks in public.
- “But I’m all for masks, I think masks are good,” the president said, adding that he has been seen wearing one in situations where he was in a group of people
- 06/30/2020 – Fed and Treasury are ready to send more stimulus relief, but the final decision depends on congress and senate
Mnuchin, Powell Pledge Additional Relief to Prevent Lasting Damage to Economy
They offer few specifics in House hearing as stimulus measures are set to expire
- 06/29/2020 – If american start to wear simple masks, then we might can see the reduction of infection like many other countries
California, Texas see record COVID-19 surges, Arizona clamps down
The city of Jacksonville, Florida, venue for part of the Republican nominating convention in August, said on Twitter it would be requiring masks in public starting later on Monday.
White House press secretary Kayleigh McEnany said on Monday that Trump “has no problem with masks and to do whatever your local jurisdiction requests.”
- 06/29/2020 – I also see the risks from the pandemic, fading fiscal stimulus, volatility around the election and worsening relations with China
BlackRock sees risks for U.S. stocks in the second half from virus and politics
- BlackRock Investment Institute is neutral on U.S. equities, or benchmark weight, going into the second half, after the U.S. market’s outperformance since late March.
- In its second-half outlook, BlackRock said it is overweight credit and it expects European equities to outperform.
- Risks to U.S. stocks include concerns about the pandemic, fading fiscal stimulus, volatility around the election and worsening relations with China.
“We’re worried about a fiscal cliff in late July around unemployment insurance and support for small businesses,” he said. Pyle said it is also unclear how much support Congress will be willing to provide for state and local governments.
“I think it’s likely we do see congressional action. The question mark: is it going to be enough? Is it going to be composed in the right way to support the economy,” said Pyle.
“With $2 trillion plus of support fiscally as well as a monetary policy response from the Fed, as we look ahead, our concern is the U.S. runs a risk in the back half of the year of retrenching too quickly on fiscal policy,” he said.
- 06/29/2020 – CV might be out of control in US? US might need a strong, united and cohesive leadership to get out the problem
CDC says U.S. has ‘way too much virus’ to control pandemic as cases surge across country
- The coronavirus is spreading too rapidly and too broadly for the U.S. to get it under control as some other countries have, Dr. Anne Schuchat, principal deputy director of the Centers for Disease Control and Prevention, said Monday.
- The U.S. stands in stark contrast to countries like South Korea, New Zealand and Singapore as it continues to report over 30,000 new infections per day.
- “This is really the beginning,” Schuchat said of the U.S.’s recent surge in new cases.
- 06/29/2020 – Relationship between US-China deteriorated due to HK, will China end the trade deal as a retaliation?
U.S. ends defense exports to Hong Kong, looking at more restrictions
WASHINGTON (Reuters) – The United States began eliminating Hong Kong’s special status under U.S. law on Monday, halting defense exports and restricting the territory’s access to high technology products as China prepares new Hong Kong security legislation.
He said the United States, effective Monday, was ending exports of defense equipment to Hong Kong and will also take steps to end the export of dual-use technologies to the territory. Dual-use technologies have both commercial and military uses.
Commerce Department regulations that gave preferential treatment to Hong Kong over China, including the availability of export license exceptions, were also suspended, Commerce Secretary Wilbur Ross said after Pompeo’s announcement.
- 06/29/2020 – the worst of CV-19 is yet to come?
- “Although many countries have made some progress, globally, the pandemic is actually speeding up,” WHO chief Tedros Adhanom Ghebreyesus said during a virtual news conference.
- The virus has infected more than 10.1 million people around the world and killed at least 502,634 people, according to data compiled by Johns Hopkins University.
- “The single most important intervention is … tracing and quarantine contacts,” he said. “Six months since the virus started, it could be like a broken record to say exactly the same thing, but the same thing works. Test, test, isolate, quarantine cases.”
“Although many countries have made some progress, globally, the pandemic is actually speeding up,” he said during a virtual news conference from the agency’s Geneva headquarters. “We all want this to be over. We all want to get on with our lives, but the hard reality is that this is not even close to being over.”
The virus has infected more than 10.1 million people around the world and killed more than 502,000 people so far, according to data compiled by Johns Hopkins University. More than 60% of daily new cases came from countries in the Americas on Sunday, according to data published by the WHO.
More than 23% of the 189,077 new cases reported globally on Sunday came from the U.S., according to the WHO’s data. Brazil was the only country in the world to report more new cases on Sunday than the U.S., according to the WHO.
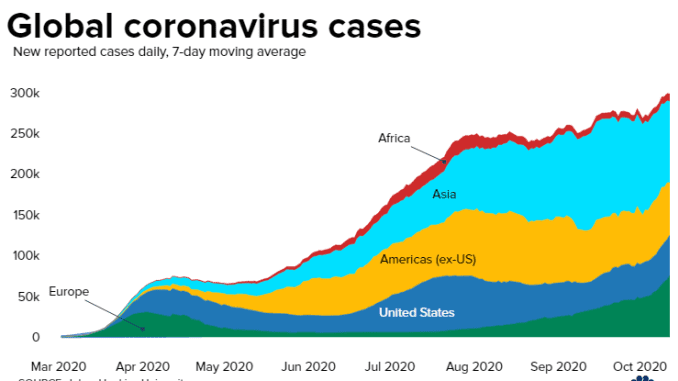
- 06/29/2020 – the next stimulus plan might come earlier?
Pelosi and Schumer urge McConnell to start bipartisan talks on more coronavirus relief as cases surge
- Nancy Pelosi and Chuck Schumer urged Mitch McConnell to immediately start talks on another coronavirus relief bill.
- McConnell has said he will consider whether to pass more legislation after sometime in July.
- Coronavirus cases continue to rise in the U.S. as millions of Americans still struggle to cover costs during the pandemic.
- 06/28/2020 – I agree with this: The course of the economy will be determined by the course of the virus
Weighing the Week Ahead: Sustaining a Fragile Recovery
New Deal Democrat’s high frequency indicators have always been a valuable part of my economic review. They are especially important as we all try to monitor the economic recovery. His three time frames all show some improvement – positive in the long-term, neutral in the short-term, and “less awful” in the nowcast. NDD has a warning, however:
…(W)hether conditions continue to improve from their recent horrible levels depends very much on the trajectory of the coronavirus pandemic. Fundamentally, this is entirely dependent on decisions made by 51 people: 50 Governors + 1 President. It is also subject to whether Congress extends the supplemental unemployment insurance payments that were temporarily enacted two months ago, but expire at the end of July. Several States in the Deep South and Southwest have started to reimpose some business closures, so continued improvement is very questionable, to say the least.
- 06/28/2020 – encourage news on vaccine from China, but it is only in Phase1/2, phase 3 has not yet started.
Chinese firm says coronavirus vaccine candidate shows promise in human test
BEIJING (Reuters) – China National Biotec Group (CNBG) said on Sunday that early human test results for a coronavirus vaccine candidate suggested it could be safe and effective, the second vaccine candidate from the firm to show encouraging results in a clinical trial.
The experimental shot, developed by a Beijing-based unit of CNBG, has induced high-level antibodies in all the inoculated participants in a Phase 1/2 clinical trial involving 1,120 healthy people, according to preliminary data of the trial, CNBG said in a posting on the social media platform WeChat, without disclosing specific readings.
Chinese companies and researchers have been allowed to test eight vaccine candidates in humans at home and abroad, making China a major front-runner in the race to develop a shot against the virus that has killed nearly 500,000 people globally.
CNBG, affiliated to the state-owned China National Pharmaceutical Group (Sinopharm), said earlier this month that another vaccine candidate produced by its Wuhan-based unit also triggered high-level antibodies safely in clinical trial participants based on preliminary results.
A vaccine has to prove its effectiveness in “Phase 3” human test where thousands of participants are recruited in order to be cleared for sale.
CNBG said on Tuesday it will run a Phase 3 for its vaccine candidate in the United Arab Emirates, without specifying which shot will be tested.
- 06/26/2020 – more pandemic might come in the future
Pandemic-proofing the planet
New diseases are inevitable. Ensuing global calamities are not
- 06/26/2020 – I need to hedge against the future catastrophe. e.g If a coronal mass ejection (cme) were to hit, all sorts of satellite systems needed for navigation, communications and warnings of missile attacks would be at risk. Large parts of the planet could face months or even years without reliable grid electricity.
Politicians ignore far-out risks: they need to up their game
Preparedness is one of things that governments are for
Asteroid strikes were an extreme example of the world’s wilful ignorance, perhaps—but not an atypical one. Low-probability, high-impact events are a fact of life. Individual humans look for protection from them to governments and, if they can afford it, insurers. Humanity, at least as represented by the world’s governments, reveals instead a preference to ignore them until forced to react—even when foresight’s price-tag is small. It is an abdication of responsibility and a betrayal of the future.
Pandemics are disasters that governments have experience of. What therefore of truly novel threats? The blazing hot corona which envelops the Sun—seen to spectacular effect during solar eclipses—intermittently throws vast sheets of charged particles out into space. These cause the Northern and Southern Lights and can mess up electric grids and communications. But over the century or so in which electricity has become crucial to much of human life, the Earth has never been hit by the largest of these solar eructations. If a coronal mass ejection (cme) were to hit, all sorts of satellite systems needed for navigation, communications and warnings of missile attacks would be at risk. Large parts of the planet could face months or even years without reliable grid electricity (see Briefing). The chances of such a disaster this century are put by some at better than 50:50. Even if they are not that high, they are still higher than the chances of a national leader knowing who in their government is charged with thinking about such things.
- 06/26/2020 – this might be the real reason US cannot contain the virus as effective as that of Europe – US Declaring victory too soon!
A Horrifying U.S. Covid Curve Has a Simple Explanation
A growing gap in case growth between Europe and the U.S. tells the tale: Declaring victory too soon is an excellent way to return to new heights.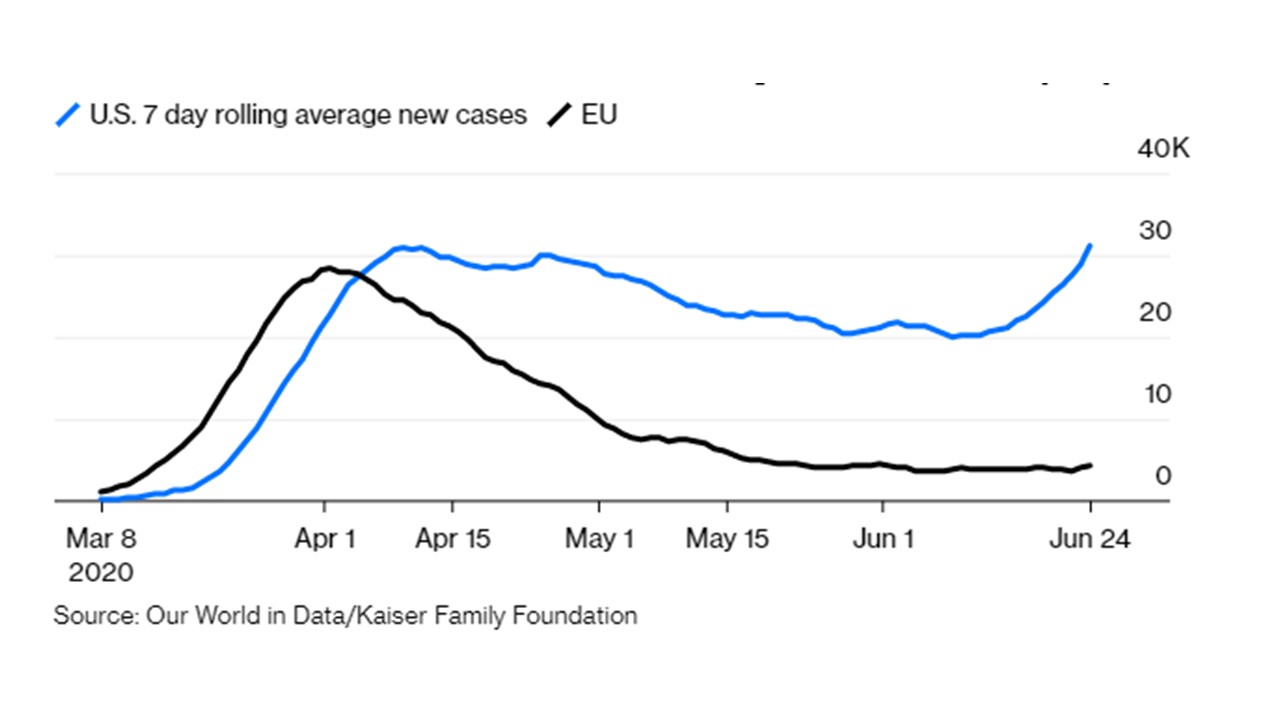
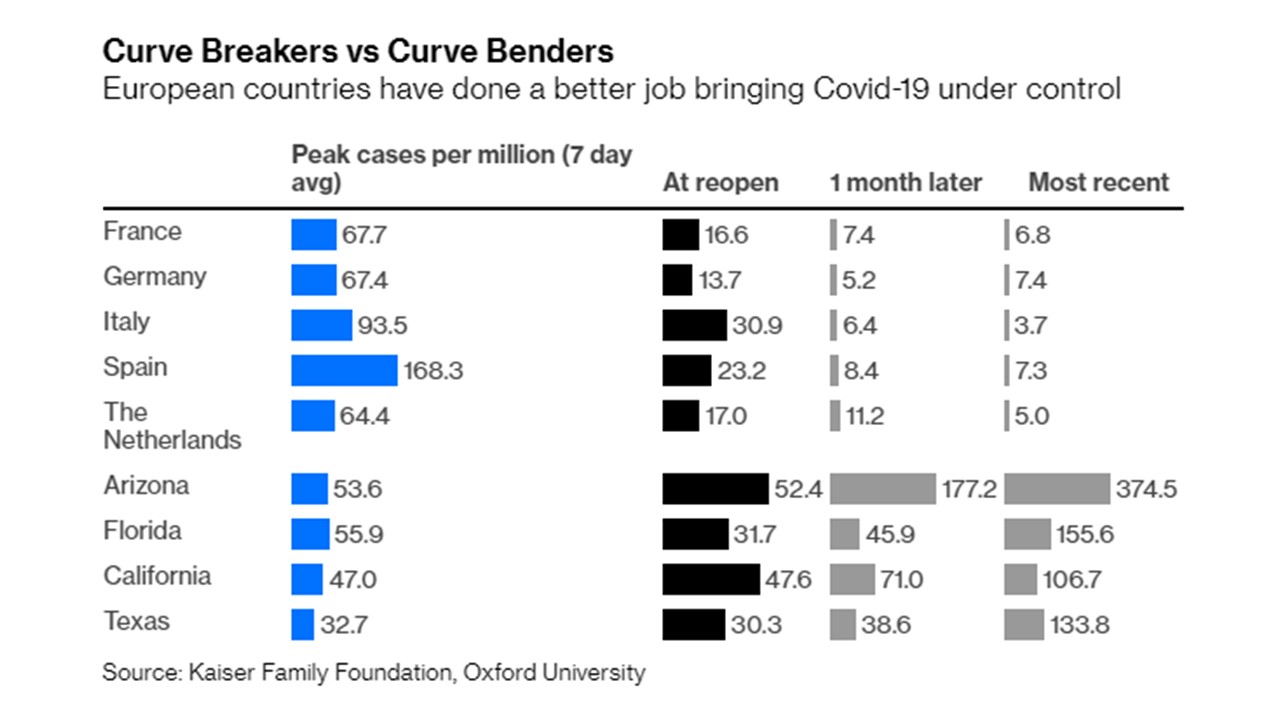
- 06/26/2020 – without more stimulus plan, market will probably plunge again.
Here comes a 20% stock market plunge if Trump and Democrats don’t agree on more COVID-19 stimulus
Add the potential of not getting another massive new fiscal stimulus plan as beginning to weigh on the minds of otherwise exuberant investors.
“I think you are looking at a 10% to 15% range minimum [if there isn’t a new stimulus plan],” warned EvercoreISI senior managing director Dennis DeBusschere on Yahoo Finance’s The First Trade. “And then from there it’s going to be about your expectation for the policy response. So you probably go down 10% to 15%, and then we’ll all wait around to see if that 10% to 15% causes the political apparatus to get moving quickly. And if you ultimately get nothing, by the way, you know it’s a down 20%. And you know, we’ll reassess from there.”
- 06/26/2020 – It is true that US-China relationship deteriorates significantly in the last three months. Lots of risks on the market.
It’s not just coronavirus: Why US-China relations are worsening on ‘almost every front’
While the president may be holding on to the message the deal is still on, Bremmer warned some in his own circle are not sold.
“The advisors I’ve spoke to in the White House, including some very senior ones, are telling me that on balance, they think the deal will not last through the election. But it’s very close,” he added.
- 06/26/2020 – AstraZeneca’s vaccine has begun 3rd stage trial. Moderna’s will start in the mid of July. So watch out their development timeline.
AstraZeneca, Moderna ahead in COVID-19 vaccine race: WHO
AstraZeneca’s (AZN.L) experimental COVID-19 vaccine is probably the world’s leading candidate and most advanced in terms of development, the World Health Organization’s (WHO) chief scientist said on Friday.
The British drugmaker has already begun large-scale, mid-stage human trials of the vaccine, which was developed by researchers at University of Oxford.
This week, AstraZeneca signed its tenth supply-and-manufacturing deal.
“Certainly in terms of how advanced they are, the stage at which they are, they are I think probably the leading candidate,” WHO chief scientist Soumya Swaminathan told a news conference.
“So it’s possible they will have results quite early.”
Swaminathan said Moderna’s (MRNA.O) COVID-19 vaccine candidate was “not far behind” AstraZeneca’s, among more than 200 candidates, 15 of which have entered clinical trials.
“We do know that Moderna’s vaccine is also going to go into phase three clinical trials, probably from the middle of July, and so that vaccine candidate is not far behind,” she said.
- 06/25/2020 – risk of China-US deal gets derail
China Message to U.S.: Crossing ‘Red Lines’ Could Put Trade Deal at Risk
Beijing quietly tells Washington that ‘meddling’ in Hong Kong, Taiwan and other matters could jeopardize Chinese goods purchases under the Phase One trade deal
- 06/25/2020 – lots of risks and uncertainty are coming in the next couple weeks
As U.S. coronavirus cases spike, country will ‘be seeing more deaths,’ Dr. Fauci says
- Deaths caused by Covid-19 lag behind other data points such as hospitalizations, which lag behind confirmed infections as the disease can take weeks to fully develop in a person.
- As cases have risen in recent weeks, new deaths have steadily decreased, but Fauci, director of the National Institute of Allergy and Infectious Diseases, warned that trend might not last for long.
- “There are more cases. There are more hospitalizations in some of those places and soon you’ll be seeing more deaths,” Fauci said in an interview with CNBC’s Meg Tirrell.
- 06/25/2020 – I agree with his take. Todd seems to also have the same opinion: in the US, contact tracing is still ramping up, many people aren’t wearing a mask or face covering, and many people are not following the 3Cs. it doesn’t take an official lockdown to push local economies back into recession – many people will pull back as the number of cases and hospitalizations rise. I do expect another round of disaster relief in July – extending the extra unemployment benefits (perhaps at a lower level), extending the PPP, and providing relief to the States. Without this disaster relief, the entire US economy might slide back into recession in August. But even with another round of disaster relief, it seems likely the recovery will stall unless progress is made in slowing the spread of the virus. The longer the widespread pandemic continues, the more structural damage to the economy. And the more severe the economic damage, the longer it will take to recover from the pandemic.
Economic Outlook: The Gathering Storm
by Calculated Risk on 6/25/2020 02:15:00 PM
On March 31st, I wrote:
This is a healthcare crisis, and the economic outlook is based on presumptions about the course of the pandemic.
I’ve been updating my outlook monthly, and turning more and more pessimistic due to the poor US government response to the pandemic.
The Federal government’s response has not been helpful, and in many ways, it has been counterproductive. The Vice President of the US, Mike Pence, wrote just last week in the WSJ: There Isn’t a Coronavirus ‘Second Wave’. The data has already proven Pence wrong.
Fortunately, some state and local governments have stepped up to fill the void, but in other localities, the response has been terrible. And it is possible the pandemic will get worse in the Fall.
Some countries have been able to open their economies without an increase in infections. For example, in Japan, the economy is mostly open, however 1) everyone wears a mask, 2) there is robust contact tracing, and 3) everyone is urged to follow the 3 C’s (avoid Closed spaces, Crowded places, and Close-Contact settings). However, in the US, contact tracing is still ramping up, many people aren’t wearing a mask or face covering, and many people are not following the 3Cs.
However, there is no leadership in the US to significantly change people’s behavior.
It doesn’t take an official lockdown to push local economies back into recession – many people will pull back as the number of cases and hospitalizations rise. Based on recent hospitalization data, I expect further layoffs in some states like Arizona, Texas, and Florida.
I do expect another round of disaster relief in July – extending the extra unemployment benefits (perhaps at a lower level), extending the PPP, and providing relief to the States. Without this disaster relief, the entire US economy might slide back into recession in August.
But even with another round of disaster relief, it seems likely the recovery will stall unless progress is made in slowing the spread of the virus. The longer the widespread pandemic continues, the more structural damage to the economy. And the more severe the economic damage, the longer it will take to recover from the pandemic.
Everyone is hoping for a vaccine in late 2020 or early 2021, but even if there is a vaccine, the damage to the economy will be extensive if we don’t lower the infection rate significantly in the near term.
Perhaps there will be a sudden change in behavior while we wait for a vaccine – or some other breakthrough that will slow the spread of the virus – but I’m not sanguine.
After a decade of making fun of bearish analysts and writing “the future is bright”, it pains me to be pessimistic. I hope I’m wrong on the virus, but if I’m correct, then I expect every major economic forecast will be revised down for the 2nd half of 2020 and for early 2021.
- 06/24/2020 – overview
Mapping the Coronavirus Outbreak Across the World
- 06/24/2020 – death at younger ages in US is alarming
When covid-19 deaths are analysed by age, America is an outlier
American casualties tend to be younger than European ones, which has grim implications

- 06/24/2020 – the 2nd wave might come in Oct, Nov.m Dec and Jan. So be ready
Explainer: What is a second wave of a pandemic, and has it arrived in the U.S.?
Dr. Theo Vos of the University of Washington’s Institute for Health Metrics and Evaluation called those assurances “wishful thinking.”
Based on global models, his group has predicted that the coronavirus will surge in the fall as colder temperatures arrive in the United States.
“It’s likely to start picking up in October,” Vos said, with increased cases hitting in November, December and January.
- 06/24/2020 – Bill thinks US reopening is sloppy because of resurge of CV-19. He wishes medicine will be somewhat ready by Fall this year. And we have better therapies than we did before.
Ackman, Rubenstein on Markets, Money and More – Bloomberg
Bill Ackman, Pershing Square Capital Management founder and chief executive officer, says the U.S. probably won’t be back to a normal economy until the second half of 2021. He speaks with Carlyle Group co-founder David Rubenstein during the Bloomberg Invest Global 2020 virtual conference. (Source: Bloomberg)
- 06/23/2020 – Fauci said the next couple of weeks are going to be critical. I need to prepare for the volatility
Fauci gives Congress COVID-19 warning
Fauci said time is running out to address the spikes in cases.
“Right now, the next couple of weeks are going to be critical in our ability to address those surgings that we’re seeing in Florida, in Texas, in Arizona and in other states,” Fauci told the House Energy and Commerce Committee Tuesday.
Both Fauci and Centers for Disease Control and Prevention Director Robert Redfield rejected that idea and said the virus will continue well into the fall and winter.
Redfield warned that the outbreak will overlap with flu season in the fall, which “could place a tremendous burden on the health care system.”
The inability of the U.S. to contain the coronavirus outbreak is being felt internationally as well. As the European Union reopens its borders, the region is reportedly considering banning all U.S. travelers given the worsening COVID-19 situation in the United States.
COVID-19, the disease caused by the virus, has killed more than 120,000 Americans.
“Cases up only because of our big number testing. Mortality rate way down!!!” Trump tweeted on Tuesday.
Yet Fauci contradicted him almost in real time, saying it was too soon to draw conclusions about the death rate.
“Deaths always lag considerably behind cases,” Fauci said. “You’re seeing more cases now while the deaths are going down. The concern is if those cases infect people who wind up getting sick and going to the hospital, it is conceivable you may see the deaths going up.”
- 06/23/2020 – the next round of stimulus plan might come in end of July
Treasury Dept. May Consider Extending Tax Filing Deadline a Second Time
Secretary Mnuchin said he hopes next round of stimulus could pass both houses of Congress in July
Mr. Mnuchin, who noted that he attended Senate Republicans’ weekly luncheon before the interview Tuesday, said his colleagues on Capitol Hill are beginning to discuss details of a fifth coronavirus-related bill to provide aid to the U.S. economy.
“We want to make sure whatever we do going forward is much more targeted to the businesses that are most impacted,” Mr. Mnuchin said. He added that he’s hopeful the next round of stimulus could pass both houses of Congress in July, when a $600 weekly supplement to workers’ unemployment benefits is set to expire.
- 06/20/2020 – Howard Marks warns the market, very reasonable. At least I think market has more downside risks than upside potential (which is already exhausted). Plus, the virus crisis suddenly turns worst and there is no sign of diminish soon.
or the link from Oaktreecapital
the three stages of a bull market. There’s a usual progression in market advances according to this beauty, and as far as I’m concerned, it’s absolutely accurate and fully captures the reality:
-
the first stage, when only a few unusually perceptive people believe improvement is possible;
-
the second stage, when most investors realize that improvement is actually taking place; and
-
the third stage, when everyone concludes everything will get better forever.
Looking back (which is the main way we know these things), the first stage began in mid-March and culminated on March 23. Certainly very few people were thinking about economic improvement or stock market gains around that time. Then we passed briefly through stage two and went straight to stage three.
Certainly by the time the interim high was reached on June 8, it felt like the market was being valued in a way that focused on the positives, swallowed them whole, and overlooked the negatives. That’s nothing but a value judgment on my part. It’s just my opinion that the imbalance of attention to – and blanket acceptance of – the positives was overdone.
- 06/20/2020 – the crowd is not that much as expected, do not know whether voters are tired of Trump or they are blocked from entering
Trump Holds Rally in Tulsa, Oklahoma | NBC News
- 06/19/2020 – I think Fauci is right on this. Virus is spreading fast now due to social unrest and business reopening (maybe) while not abiding the opening guidelines! To make things worse, leaders are reckless.
Fauci: Americans ignoring science during pandemic is “frustrating”
Dr. Anthony Fauci, the nation’s leading infectious diseases expert, is frustrated about the nation’s inability to stop the continued spread of coronavirus, which he says is largely because Americans aren’t following recommended health guidelines.
With the infection still active and rising in parts of the country, Fauci also discussed the “very clear” risk that coronavirus can spread at mass gatherings like President Trump’s rally in Oklahoma on Saturday, his first since early March. He advises staying away.
“The best way to protect yourself and to prevent acquisition of and spread of infection is to avoid crowds. Avoid crowds. If in fact, for one reason or other, you feel compelled to do that, which we don’t recommend, then wear a mask at all times,” Fauci told Portnoy. “So when you see situations, when people are not doing — that they are in crowds and or they’re not wearing masks when they’re outside, of course, that gives us concern about the increased risk of spreading infection.”
The campaign is going to provide attendees with hand sanitizer, temperature checks and masks upon entry, but wearing a mask will be optional. The venue, the Bank of Oklahoma Center, holds 19,000 people, and an additional stage in an outdoor area adjacent to the venue holds several thousand more.
And he blames the inconsistent public response for the recent “burst of infections.”
Top members of coronavirus task force advised against Trump’s Tulsa rally
- 06/17/2020 – may be even though with CV-19 second wave, US will not lock down again?
Trump says U.S. will not lock down again amid rising coronavirus cases
- 06/17/2020 – fed again urges for more stimulus measures
Powell Says Economic Gains Are at Risk if Stimulus Measures End Prematurely
‘It would be a concern if Congress were to pull back…too quickly,’ Fed chair tells lawmakers
- 06/17/2020 – Trump’s infrastructure plan hits snag. Given so divided congress and senate, it is not a smooth path. I should not bet on it too soon and put too much money on it. Wait for the dip. Sell the news.
Trump’s infrastructure plan hits snag among Senate Republicans
Senate Majority Leader Mitch McConnell (R-Ky.) has expressed concerns about adding to the national debt that has grown because of the coronavirus pandemic and is leery about passing another financial stimulus plan.
Republican senators have warned that Trump’s plan is too “rich” and would require a “heavy lift” in Congress to bridge the policy differences with the House Democrats’ $500 billion transportation bill, The Hill reported.
The House Transportation Committee is scheduled to begin debate on the Democrats’ bill Wednesday.
McConnell’s priority, GOP senators said, is to push for a five-year reauthorization of the Highway Trust Fund, which is estimated to cost $287 billion, far below Trump’s $1 trillion requested amount.
Sen. Chuck Grassley (R-Iowa), chairman of the Senate Finance Committee, said his panel is still trying to figure out how to cover $93 billion in the reauthorization bill.
Grassley, when asked about Trump’s proposal, said whatever GOP senators come up with “could be a lot less.”
Sens. Pat Toomey (R-Pa.) and Mike Enzi (R-Wyo.), both members of the finance committee, were skeptical about authorizing another large spending measure.
“I think that’s a very heavy lift,” Toomey said. Enzi, chairman of the Senate Budget Committee, said the focus should still be on the coronavirus pandemic and noted that much of the $2 trillion CARES Act and the $484 billion interim coronavirus relief legislation still hasn’t been spent.
“For the last few days I’ve been talking about not paying for the national parks’ infrastructure. A trillion is a lot more than the $17 billion we’re talking about there,” he said, referring to the Great American Outdoors Act. “I’m much more inclined to stick to solving the virus problem.”
Sen. John Cornyn (R-Texas) questioned why the Trump administration put a price tag on the infrastructure proposal before debating what’s in it.
“You don’t start with the price tag. You start with what it is you want to accomplish and figure out what that is. Seems to me to be the opposite way to approach this by starting it with how much money you’re willing to spend,” Cornyn said.
Sen. Rob Portman (R-Ohio) balked at the cost, saying a “trillion dollars may be a little rich.” But he also said there are parts that he likes.
At least four Congress members reportedly benefited from stimulus loans
“I think there are areas where we can do something. Rural broadband is very popular among many of my colleagues,” Portman said. According to a report Tuesday, the Department of Transportation is spearheading Trump’s plan that would spend funds on 5G wireless infrastructure and rural broadband, as well as traditional roads and bridges.
Sen. Lisa Murkowski (R-Alaska) said she’d be open to considering an infrastructure plan, and blamed a lack of communication between the Senate and the House for its lackluster welcome.
- 06/16/2020 – this is a good but not too big news
HEALTH AND SCIENCE
‘Breakthrough’ coronavirus treatment dexamethasone is a ‘good first step,’ lead researcher says
- 06/16/2020 – am I too optimistic on this? I need to be more patient
It’s ‘unrealistic’ for investors to bet on Trump administration’s $1 trillion infrastructure plan, analyst says Published: June 16, 2020 at 2:15 p.m. ET
Divided Washington could struggle to deliver a big infrastructure package before November elections
Next coronavirus aid package to pass Congress by late July, analysts say
Published: June 1, 2020 at 2:03 p.m. ET
‘The social unrest over the past weekend will probably lead Democrats to push for more funds for states and local governments,’ one analyst says
- 06/15/2020 – this would be huge if it comes, why did I forget about it? Following news might come on Wednesday and Thursday (06/17, 06/18)
Dow futures surge amid report that Trump is preparing $1 trillion infrastructure proposal
Trump Team Weighs $1 Trillion for Infrastructure to Spur Economy
The Trump administration is preparing a nearly $1 trillion infrastructure proposal as part of its push to spur the world’s largest economy back to life, according to people familiar with the plan.
A preliminary version being prepared by the Department of Transportation would reserve most of the money for traditional infrastructure work, like roads and bridges, but would also set aside funds for 5G wireless infrastructure and rural broadband, the people said.
President Donald Trump is scheduled to discuss rural broadband access at a White House event on Thursday.
The Democratic bill to reauthorize the current infrastructure program was unveiled this month. It includes investments in roads and bridges, funding to make certain projects more resilient to climate change, and funding for public transit and Amtrak, among other priorities. The House Transportation committee is set to take up the measure on Wednesday.
The existing surface transportation authorization law, known as the FAST Act, authorizes $305 billion over five years and expires on Sept. 30. Lawmakers will either extend it or come up with a long-term replacement. It’s not yet clear how closely the administration’s plan will align with the Democrats’ proposal — or with what Senate Majority Leader Mitch McConnell might do.
- 06/15/2020 – it is amazed to see the market comes back 1000 points in one day. I can see clearly how much profit I can make if I had good portfolio management (only small portion of LEAP)!!!!!!!!!!!!! If I had good (peace of mind) portfolio allocation, I will buy dip in the early morning market. Remember, Trump, Fed and Treasury always have some tools to (short term) beef up the stock market.
Stocks Close Higher After Falling on Virus Fears
Dow recovers after the Federal Reserve followed through on moves to support corporate bonds
- 06/15/2020 – this will add in volatility of market
New Covid-19 Outbreak in China Rattles Beijing
Residents of capital had started to relax after weeks without new locally transmitted cases; ‘Oh my God, again’
- 06/11/2020 – Need to understand its impact on economy and market
Investors Get Ready for the Fed to Cap Rates
In suppressing yields, the market could lose an important benchmark against which much of the investment universe is measured
Brisk Jobs Growth Puts Focus on Fed
With unemployment in central bank’s target zone, stage is set for interest-rate increases
Steven Mnuchin Says White House Considering Second Round of Stimulus Payments
Treasury secretary noted it is premature to speculate on overall size of next relief package
- 06/09/2020 – No rate hike till 2022.
Fed Officials Project No Rate Increases Through 2022
Central bank says it will maintain current pace of asset purchases to sustain growth
link to Federal Open Market Committee statement. read the pdf full document here. (This is a comparison of Wednesday’s Federal Open Market Committee statement with the one issued after the Fed’s previous policy-making meeting on April 29. Text removed from the April statement is in red with a horizontal line through the middle. Text appearing for the first time in the new statement is in red and underlined.Black text appears in both statements.)
Fed sees interest rates staying near zero through 2022, GDP bouncing to 5% next year
- 06/08/2020 – asymptomatic spread of CV is rare, so should be focus on symptomatic spread
Asymptomatic spread of coronavirus is ‘very rare,’ WHO says
- Government responses should focus on detecting and isolating infected people with symptoms, the World Health Organization said.
- Preliminary evidence from the earliest outbreaks indicated the virus could spread even if people didn’t have symptoms.
- But the WHO says that while asymptomatic spread can occur, it is “very rare.”
“We have a number of reports from countries who are doing very detailed contact tracing,” she said. “They’re following asymptomatic cases. They’re following contacts. And they’re not finding secondary transmission onward. It’s very rare.”
- 06/07/2020 – it’s no longer a question of when things start to improve, the question now is how fast they will improve. watch out the risks from election uncertainty and Fed raises int rate.
For many, the virus may even be losing its potency. Fewer people are testing positive for COVID-19 and those who test positive don’t seem to be getting as sick, a UPMC doctor said Thursday. “All signs that we have available right now show that this virus is less prevalent than it was weeks ago,” said Dr. Donald Yealy, the chair of emergency medicine at UPMC. “Your risk of getting into a car accident if you go back and forth across the turnpike in Pennsylvania is greater than your risk of being positive for asymptomatic COVID-19 infection,” he said. “This should give you some reassurance that the risk of catching COVID-19 … from someone who doesn’t even know they have the infection, in our communities, is very small.”
Yealy said he doesn’t know exactly why the prevalence and severity of COVID-19 seems to have fallen. He said it likely reflects an interplay of things including weather, possible genetic changes in the virus, people watching themselves more closely for symptoms, and better medical decisions and treatment.
So, as investors we need to forget about Covid-19, the shutdown, and the economy, since these are all on the mend; it’s no longer a question of when things start to improve, the question now is how fast they will improve.
Looking ahead, the critical areas of concern, in my mind, are 1) can Trump recover from his currently depressed levels of approval and go on to beat Biden in November, thus avoiding the economically-disastrous policies (e.g., higher taxes, increased regulation, and multiple “green” initiatives) that Biden is proposing? and 2) can the Fed react to the dramatic improvement in the economy—and the rebound in confidence which is sure to follow—by raising interest rates in a timely fashion and thereby averting an unexpected surge in inflation?
I have already begun to address this second question here, here, and here, and at the bottom of this post, but more observation is clearly needed. I will begin to address the first question in the months to come.
- 06/05/2020 – biggest job increase indicates the resilient of US economy. The biggest job gain (in decent order) come from Leisure and hospitality workers, Construction, Education and health services, personal and laundry services, and manufacturing jobs. So these industries are coming back quickly.
May sees biggest jobs increase ever of 2.5 million as economy starts to recover from coronavirus
- Nonfarm payrolls rose by 2.5 million in May and the unemployment rate fell to 13.3%.
- Wall Street estimates had been for a decline of 8.3 million and a jobless level of 19.5%, which would have been the worst since the Great Depression era.
- This was well above consensus expectations of 8,250,000 jobs lost, however March and April were revised down by 642,000 combined. This was a surprising employment report since all other data pointed to more job losses in May. The consensus is for 8,250,000 jobs lost, and for the unemployment rate to increase to 19.7%.
- Much of the gain came from those classified as temporary layoffs due to the coronavirus-related economic shutdown.
- Leisure and hospitality represented almost half the jobs gained.
Leisure and hospitality workers made up almost half the increase last month, with 1.2 million going back to work after a reported loss of 7.5 million in April. Jobs in bars and restaurants increased by 1.4 million as states began to relax social distancing measures.
Construction was the next biggest gainer with 464,000, making up for about half of April’s losses. Education and health services rose by 424,000 and retail surged by 368,000 after plunging by 2.3 million a month previous.
The other services category rose by 272,000 thanks largely to a jump of 182,000 for personal and laundry services. Coming after a decline of 1.3 million positions, manufacturing jobs increased by 225,000 despite broader signals that the sector is still in contraction.
- 06/05/2020 – Trump has strong incentive to promote the vaccine, so it might be partially true, and partially good wishes. Still lots of uncertainty
Trump says U.S. has 2 million coronavirus vaccine doses ‘ready to go’
President Donald Trump said Friday the U.S. has already produced 2 million coronavirus vaccine doses that are “ready to go” once scientists figure out whether it is safe and effective.
“Tremendous progress is being made on vaccines,” Trump said during a Friday morning press conference from the White House. “In fact, we’re ready to go in terms of transportation and logistics. We have over 2 million ready to go if it checks out for safety.”
Trump said “we’re doing incredibly well” on vaccines, adding, “I think you’re going to have some very positive surprises and therapeutics likewise we’re doing extremely well. Cures, we’re doing well.”
The Trump administration has selected five companies as the most likely candidates to produce a vaccine for the coronavirus, according to the New York Times. He didn’t say Friday which ones have started vaccine production.
The National Institutes of Health has been fast-tracking work with biotech firm Moderna on a potential vaccine to prevent Covid-19. White House health advisor Dr. Anthony Fauci said earlier this week that there are at least four trials for potential vaccines that he is either directly or indirectly involved in.
Because of the pandemic, U.S. health officials and researchers have been accelerating the development of vaccines candidate by investing in multiple stages of research even though doing so could be for naught if the vaccine ends up not being effective or safe.
U.S. officials and scientists are hopeful a vaccine to prevent Covid-19 will be ready in the first half of 2021.
- 06/04/2020 – Projections of CV-19 in US. My simple projection is similar to that of IHME (using curve fitting like what I do) and LANL (using conventional seir method), in which deaths to drop to none by July, while Youyang Guo’s model (using machine-learning algorithms to instruct a seir model – so far the most accurate model) predicts persistence even in August.
Early projections of covid-19 in America underestimated its severity
By luck or by design, they have improved markedly since
With better data and improved models, the forecasts made today should prove more accurate—either by luck or design—than earlier ones. Yet future estimates remain widely divergent. The ihme expects total deaths to stabilise in July at 140,000, whereas Mr Gu expects the virus to persist into August, at which time there will have been nearly 200,000 deaths. Either total would mean that covid-19 would have exacted a truly terrible toll.
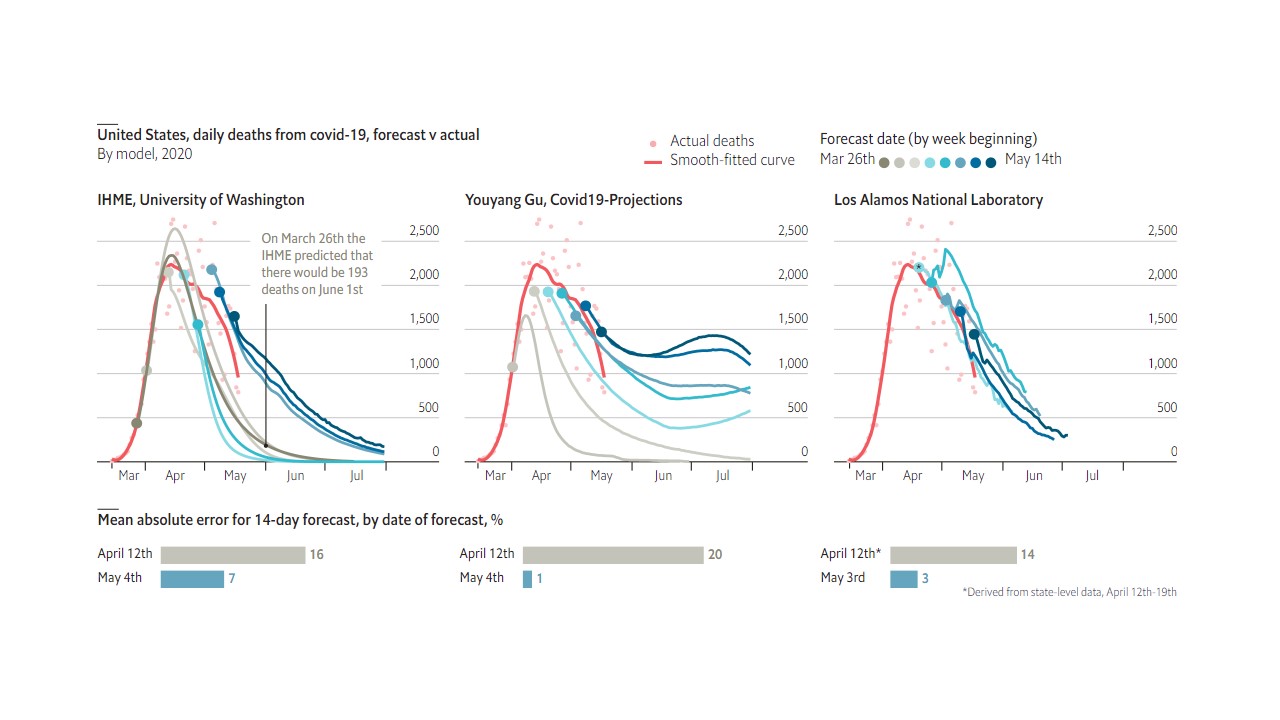
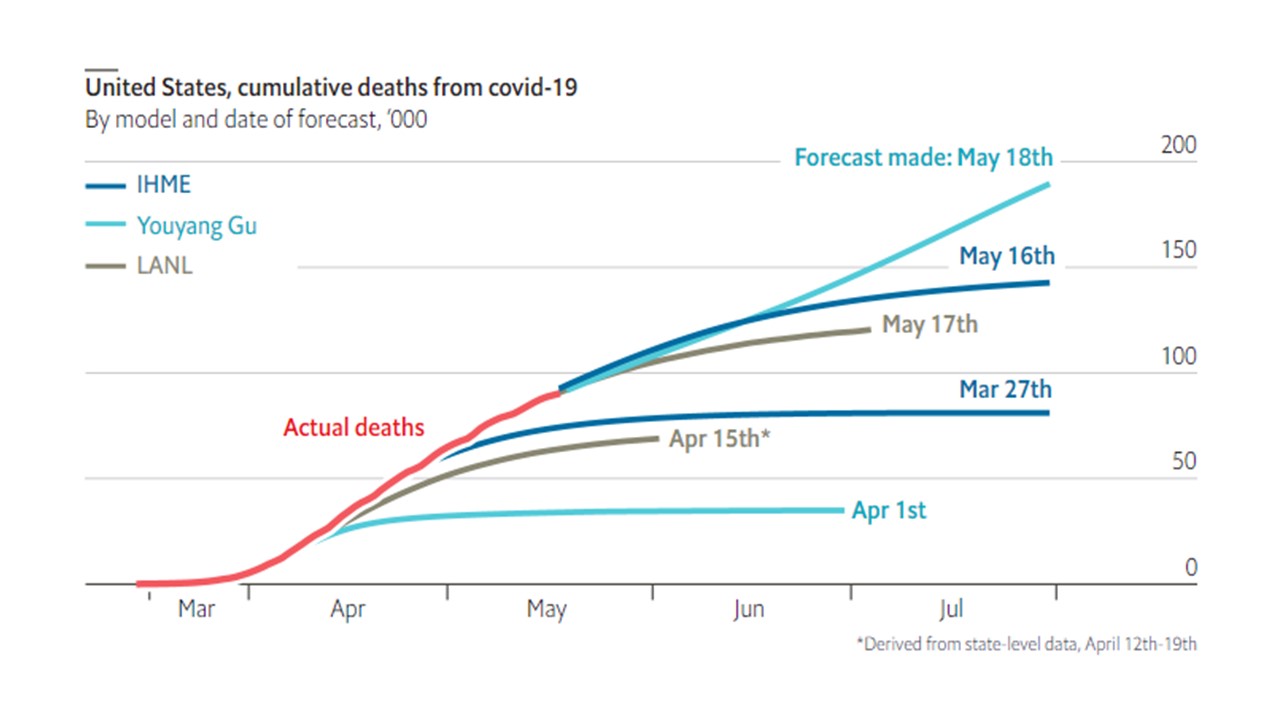
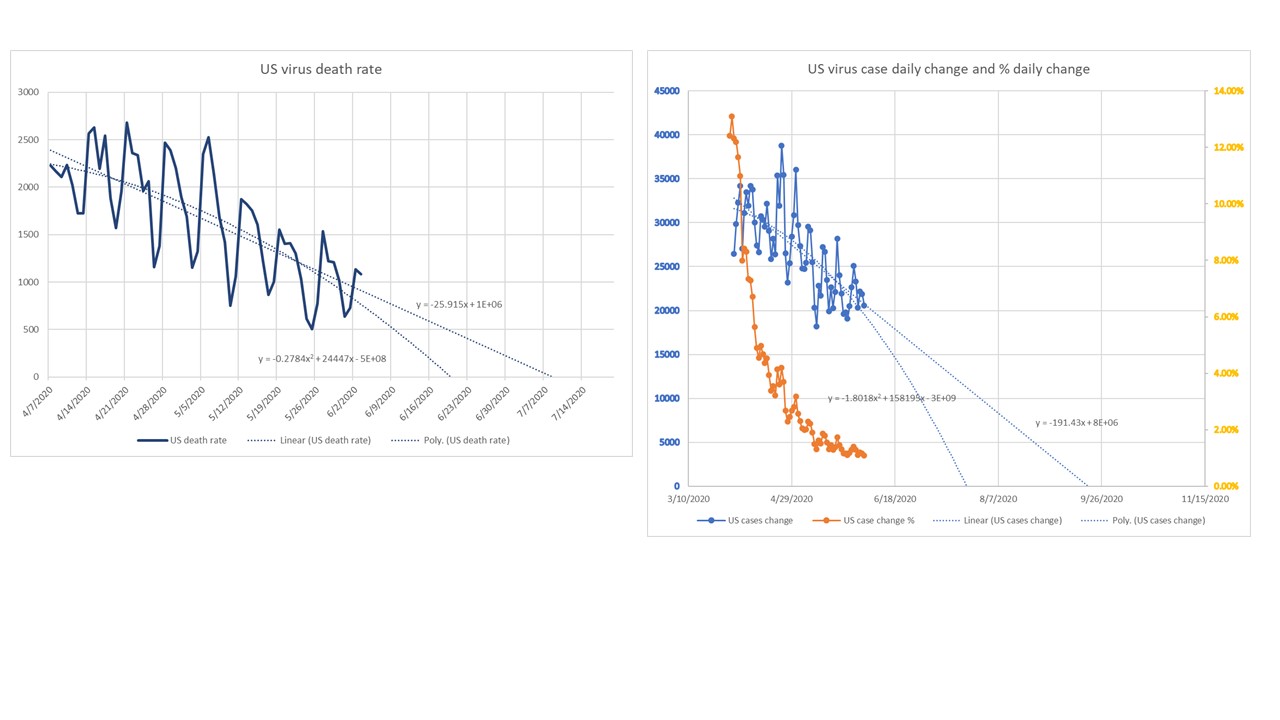
- 06/03/2020 – China economy is recovering but slowing due to less oversea customers
China’s Barely Begun Economic Recovery Shows Signs of Stalling
More factories are reopening, but they face falling orders from overseas customers
While China’s official PMI, released by the National Bureau of Statistics on Sunday, showed continued expansion, the magnitude of the gains fell for a second straight month, and a subindex to measure production slipped to 53.2 from 53.7 in April—pointing to sluggish demand. Worryingly, the new-export-orders subindex, a gauge of external demand, continued to remain deep in contractionary territory, though it improved to 35.3 in May, from 33.5 in April.
Meanwhile, the Caixin PMI survey, which is tilted toward smaller private manufacturers, showed new export orders contracting at a historically sharp rate, Caixin Media Co. and research firm IHS Markit reported Monday.
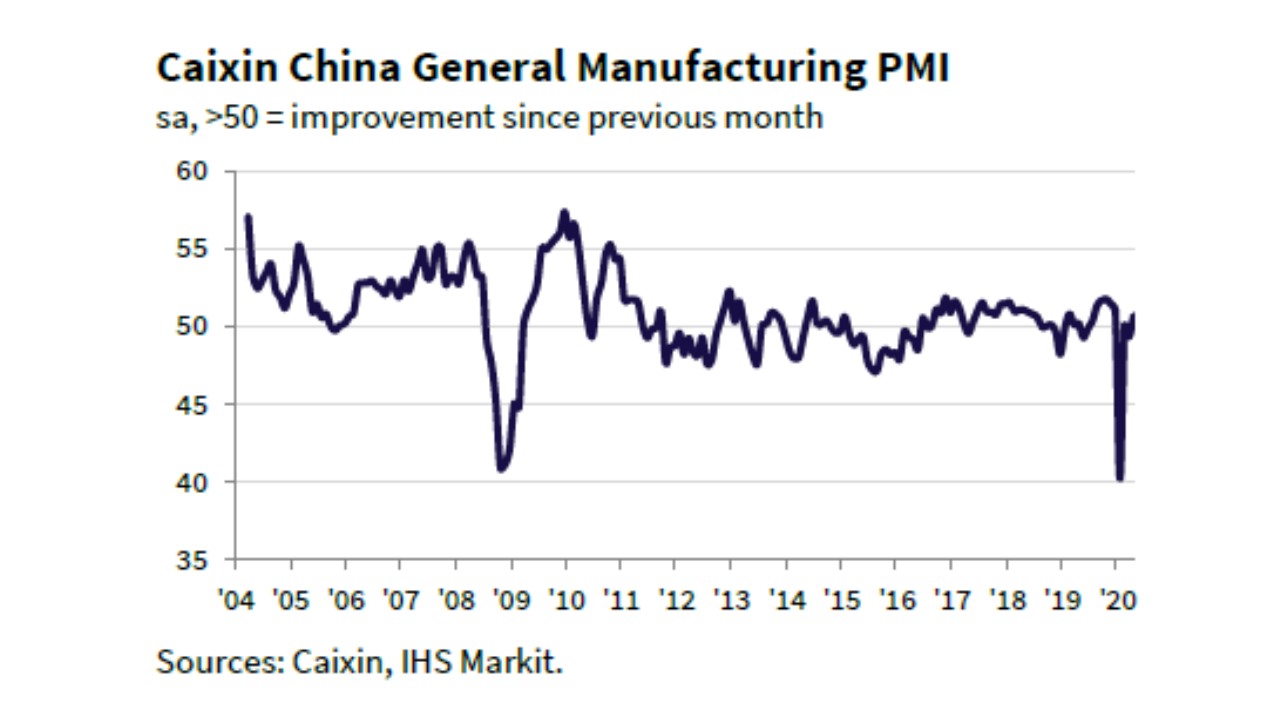
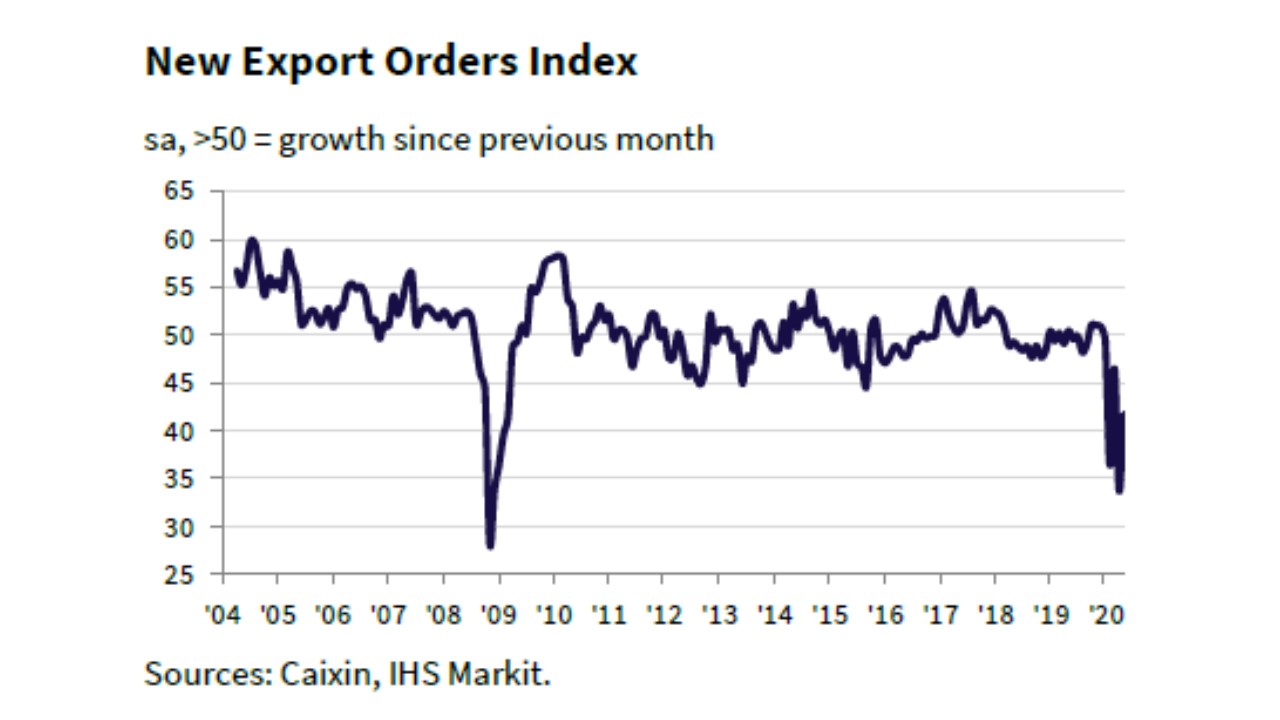
- 06/02/2020 – the next stimulus plan of maybe $1 tri is also coming from WH. White House officials are continuing to bet on a V-shaped recovery in which the economy rebounds this summer and continues growing briskly into next year, officials said.
White House Weighs Options for Next Stimulus Bill
Administration wants to encourage Americans to go to restaurants and take vacations, while congressional Democrats have their own priorities
WASHINGTON—President Trump is planning to meet with his senior advisers as soon as this week to discuss policy options for the next coronavirus relief package as the administration prepares for negotiations with Capitol Hill, according to a senior administration official.
The president’s team has assembled a set of proposals meant to encourage the public to return to work and resume normal life, including going out to restaurants and taking vacations, in an effort to jump-start the ailing economy as quickly as possible.
“We’ve been through the rescue phase and we’re now in the transitional reopening phase and I think generally speaking we’d like to move into a growth- incentive phase for the future economy,” the senior administration official said.
The timing of the meeting, which is planned for this week, could change, officials cautioned, noting that the White House is increasingly focused on the unrest around the country over racial inequality and police brutality.
Mr. Trump and his advisers have been taking a wait-and-see approach to the next round of coronavirus relief after Congress approved more than $3.3 trillion in aid in recent months, raising concerns among some of the president’s advisers about surging budget deficits. But White House officials said they aren’t trying to stall in hopes of killing the next bill.
White House officials are continuing to bet on a V-shaped recovery in which the economy rebounds this summer and continues growing briskly into next year, officials said. While many economists have projected economic activity will begin to pick up over the coming months, they see a long, slow recovery ahead. The Congressional Budget Office said Monday it could take nearly a decade for the U.S. economy to fully recover from the pandemic and related shutdowns.
- 06/01/2020 – Money-market funds have $1.2 trillion in cash; equities as % of total equities/bonds/M2 by non-bank investors is below average; SP500 futrues has the biggest net short since 2015. All these might indicate an early start of bull market run.
JPMorgan’s Math Shows Why U.S. Stocks Can Keep Rallying
-
Money managers are holding plenty of cash on the sidelines
- JPMorgan says equity allocations are still historically low
Think the sizzling U.S. stock rally is excessive in an economy frozen by shutdowns? From one perspective, it’s just getting started.
All that shows how much firepower investors have to support the market at a time when stock prices look unhinged from fundamentals like corporate profits, and trade frictions between China and the U.S. return to the forefront.
“Investors are still underweight equities and signs of overextension are confined to momentum traders,” JPMorgan strategists led by Nikolaos Panigirtzoglou wrote in a note. “There is still plenty of room for investors to raise their equity allocations.”
Another sign of cautious sentiment: investors are deeply short the market, so there’s potential for stocks to rally when they cover their positions.
Speculators have built up the largest net short position on S&P 500 futures since late 2015, according to regulatory data. Short interest in the world’s largest exchange-traded fund — which tracks the U.S. stock benchmark — is also still hovering close to its peak in March, according to Markit data.
As the bank’s strategists led by Michael Hartnett put it succinctly: “Positioning still bearish, policy bullish.”
So who’s buying? Certain breeds of quants, for one. Momentum traders, like commodity trading advisers, are the only overextended part of Wall Street, according to JPMorgan.
06/01/2020 – death of CV might become zero on June 16 ~ July 7, numbers of new cases might drop to zero on July 15 ~ Sept 15.
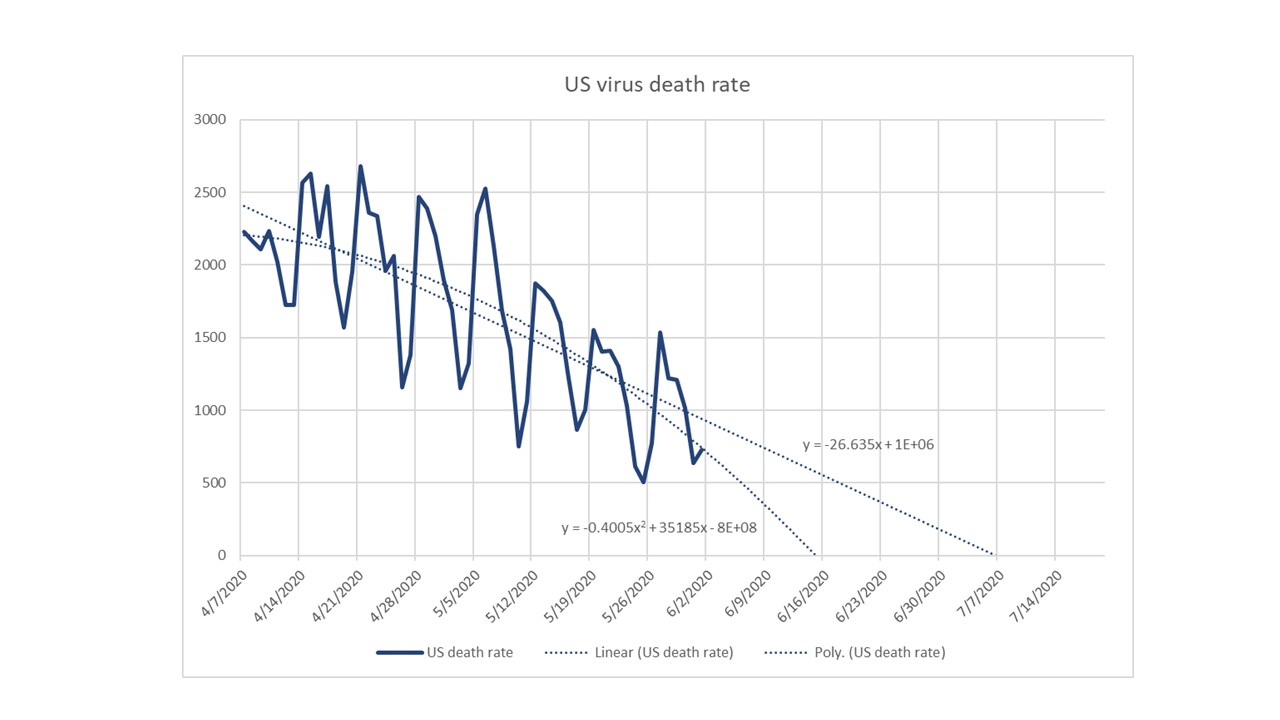
- 06/01/2020 – China is using trade deal purchase to retaliate US’s upcoming sanction on Hong Kong. Not good for US economy. Not good for Trump’s reelection.
‘Lemon’ or not, Trump stuck with Phase 1 China trade deal for now
WASHINGTON (Reuters) – U.S. President Donald Trump has little choice but to stick with his Phase 1 China trade deal for now despite his anger at Beijing over the coronavirus pandemic, new Hong Kong security rules, and dwindling hopes China can meet U.S. goods purchase targets, people familiar with his administration’s deliberations say.
Talking tough on China and criticizing the Obama administration’s more measured approach is a key part of Trump’s re-election strategy. Sticking with the pact may mean accepting that China is likely to fall short of purchase commitments for U.S. agricultural goods, manufactured products, energy and services – goals that many said were unrealistic here even before the pandemic.
In response to Trump’s Hong Kong announcement, China told state-owned firms to suspend large-scale farm purchases including soybeans and pork, people familiar with the matter told Reuters.
China orders firms to stop buying U.S. farm goods Posted June 1, 2020
China has told state-owned firms to halt purchases of soybeans and pork from the United States, sources tell Reuters, after Washington said it would eliminate special treatment for Hong Kong to punish Beijing. Fred Katayama reports.
- 05/31/2020 – everything on CV-19
What We Know About Coronavirus Tests, Treatment and Vaccines
How the U.S. is faring in the public-health race to safely reopen schools, businesses and daily life
- 05/29/2020 – econ calendar for next week
technology’ is at risk in China since companies like Apple have large revenue exposure in addition to supply chain issues. In addition to the jobs report, there is important ISM manufacturing data Monday and auto sales for the month of May.
Week ahead calendar
- Monday: 9:45 a.m. Manufacturing PMI; 10:00 a.m. ISM manufacturing; 10:00 a.m. Construction spending
- Tuesday: Monthly vehicle sales
- Wednesday: 8:15 a.m. ADP employment, 9:45 a.m. Services PMI, 10:00 a.m. ISM nonmanufacturing, 10:00 a.m. Factory orders
- Thursday: 8:30 a.m. Jobless claims, 8:30 a.m. International trade, 8:30 a.m. Productivity and costs
- Friday: 8:30 a.m. Employment, 3:00 p.m. Consumer credit
- 05/29/2020 – next stimulus plan is coming and it might can offset the US-China tension. Very positive for the market
Next coronavirus stimulus bill will be the ‘final’ one, Mitch McConnell says
- 05/29/2020 – big news on Trump’s announcement. Watch out the future reaction from China
Trump taking action to eliminate special treatment for Hong Kong
- President Donald Trump announced he would begin taking steps to revoke Hong Kong’s favored trade status with the United States.
- “My announcement today will affect the full range of agreements that we have with Hong Kong, from our extradition treaty, to our export controls and technologies,” Trump said.
- Financial markets reacted positively to Trump’s announcement, reflecting overall relief that Trump did not announce more drastic and punitive measures.
- Trump also said he was ready to take action to mandate that Chinese and other foreign companies listed on U.S. financial exchanges abide by American accounting and audit standards. The issue has long been a source of frustration among Washington policymakers, several of whom have introduced legislation that would bar trading in any shares where the company’s auditor hasn’t faced an inspection from the Public Company Accounting Oversight Board for three consecutive years. Trump has not said whether he will sign the bill, which is currently making its way through Congress.
- 05/28/2020 – political volatility arises due to US-China tensions. Need to watch out for potential opportunities
Stocks Turn Lower on China Tensions
Dow drops in final hour as Trump says he will hold a press conference regarding China and Hong Kong
- 05/28/2020 – the death rates of nursing home from CV-19 is about 50%. So the total real death numbers of other people, excluding nursing home people, is about 50k. Half of current number.
- 05/27/2020 – watch out the record monetary expansion which might drive significant inflation down the road
Monetary expansion Argentina-style
We are definitely sailing in uncharted monetary waters. In all of US history we’ve never come even close to today’s monetary environment. Chart #1 is one that no economist ever expected to see coming out of the US: Chart #1
- 05/26/2020 – Trump admin is quickly developing an international strategy for quickly developing therapeutics against the virus. The COVID-19 vaccine race officially goes into high gear with the announcement of USA’s plan to test the 6-odd top vaccine candidates across upwards of 150,000 volunteers (min. 100,000 pooled from VA hospitals and through participating pharmaceutical company recruitment efforts) in the coming months. Great news for the market
TRUMPDESIVIR: OPERATION WARP SPEED, “ENGAGE.”
Operation Warp Speed:
The COVID-19 vaccine race officially goes into high gear with the announcement of USA’s plan to test the 6-odd top vaccine candidates across upwards of 150,000 volunteers (min. 100,000 pooled from VA hospitals and through participating pharmaceutical company recruitment efforts) in the coming months. The goal is to prove out both efficacy and safety in record time, with the lead candidates ferreted out by the end of 2020. The public-private funded ACTIV is a key driver of the initiative.
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV):
(ACTIV) has identified four priorities in its effort to develop an international strategy for quickly developing therapeutics against the virus:
- Develop a collaborative, streamlined forum to identify preclinical treatments
- Accelerate clinical testing of the most promising vaccines and treatments
- Improve clinical trial capacity and effectiveness
- Accelerate the evaluation of vaccine candidates to enable rapid authorization or approval
Partners include 18 biopharmas, up from 16 when ACTIV was first announced on April 17. Eisai and Gilead Sciences have joined a group of participating companies that included J&J and many other global biopharmas: AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Evotec, GlaxoSmithKline (GSK), KSQ Therapeutics, Merck & Co., Novartis, Pfizer, Roche and its Genentech subsidiary, Sanofi, Takeda Pharmaceutical, and Vir Biotechnology.
- 05/26/2020 – US will pay companies to move back to US: China is making mistake in Hongkong. SEC requires all Chinese companies abide by the US transparency and accounting requirements. Penalty will come if China does not change.
US will pay for companies to bring supply chains home from China: Kudlow | Fox Business
- 05/26/2020 – McConnell from GOP again signals the upcoming relief plan. I need to get fully ready for it.
Congress will ‘probably’ have to pass another coronavirus stimulus bill, Mitch McConnell says
- Senate Majority Leader Mitch McConnell said Congress will “probably” have to pass another coronavirus relief bill and will decide whether to do so in the coming weeks.
- Democrats passed a $3 trillion rescue package earlier this month, but Senate Republicans have no plans to pass it.
- McConnell wants liability protections for businesses in the bill and suggests he could support limited relief for state and local governments.
- 05/20/2020 – More relief will be more positive for FAS, NAIL and TNA in the short term. So get ready for it. They hope the next Senate relief bill could pass in June under an “optimistic” scenario and predicted it would likely have to pass by the August recess, which is scheduled to begin Aug. 8.
GOP senators: More relief needed now
Senate Republican support for moving the next coronavirus relief bill as soon as next month is growing after Federal Reserve Chairman Jerome Powell warned lawmakers this week that the economic damage caused by the pandemic could last for years.
Senate Majority Leader Mitch McConnell (R-Ky.) has put the brakes on further coronavirus relief negotiations, citing the budgetary impact of trillions of dollars in unanticipated spending.
But a growing group of GOP senators, which includes some of the conference’s most vulnerable members in this year’s elections, say they shouldn’t let another month pass without significant progress on another economic relief package.
Senate Commerce Committee Chairman Roger Wicker (R-Miss.), Sen. Susan Collins (Maine), one of the chamber’s most vulnerable GOP incumbents, Sen. Cory Gardner (R-Colo.), another vulnerable incumbent, Sen. Josh Hawley (R-Mo.) , Sen. Roy Blunt ROY DEAN BLUNT a member of the Senate GOP leadership, said the next Senate relief bill could pass in June under an “optimistic” scenario and predicted it would likely have to pass by the August recess, which is scheduled to begin Aug. 8. He also joined Wicker in calling for the next relief package to include a significant infrastructure spending component. “Personally, I’d like to see infrastructure,” he said. “If we’re going to have to spend money to reignite the economy, I’d prefer to spend it on something that has long-term benefit.”
One thing that doesn’t appear to be factoring into the GOP decisionmaking process is the $3 trillion relief bill passed by House Democrats last week. Wicker said Congress needs to “tweak” aid that was given to airlines in the $2.2 trillion CARES Act, as well as the $669 billion appropriated for small businesses in the Paycheck Protection Program. “We need to look long-term at the defense industrial base and we need to seriously consider putting part of the infrastructure bill in the next phase because those are real jobs, at the end of the day you have a real asset for the money,” he added.
- 05/20/2020 – extrapolating current CV data, it seems like the new additional CV cases will become zero between 06/15 ~09/15/2020. Therefore, bet on mid of June to beginning of Oct might be a good idea.
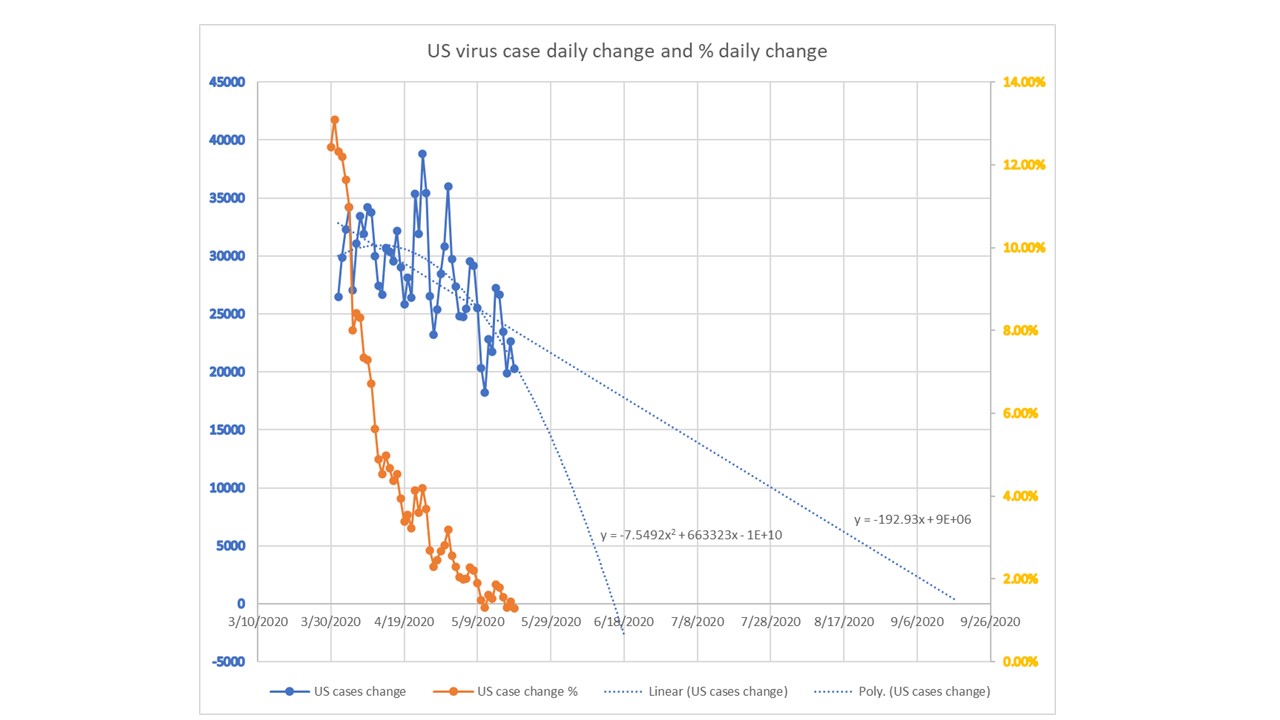
- 05/19/2020- demand in China is returning 2x faster than expectation, consumption is lagging. Need government to make more use of fiscal policy to maintain and stimulate economic growth. US might follow similar trajectory for recovery
Coronavirus Seemingly Tamed, Chinese Economy Starts to Recover. But weak sales at stores and a stall in export orders raise fears of a possible second downturn this summer. Excerpt:
Industrial production surged last month in China more than twice as fast as most economists expected, according to official data released on Friday by the country’s National Bureau of Statistics. But retail sales fell even more sharply than anticipated, while orders for future exports from China have stalled.
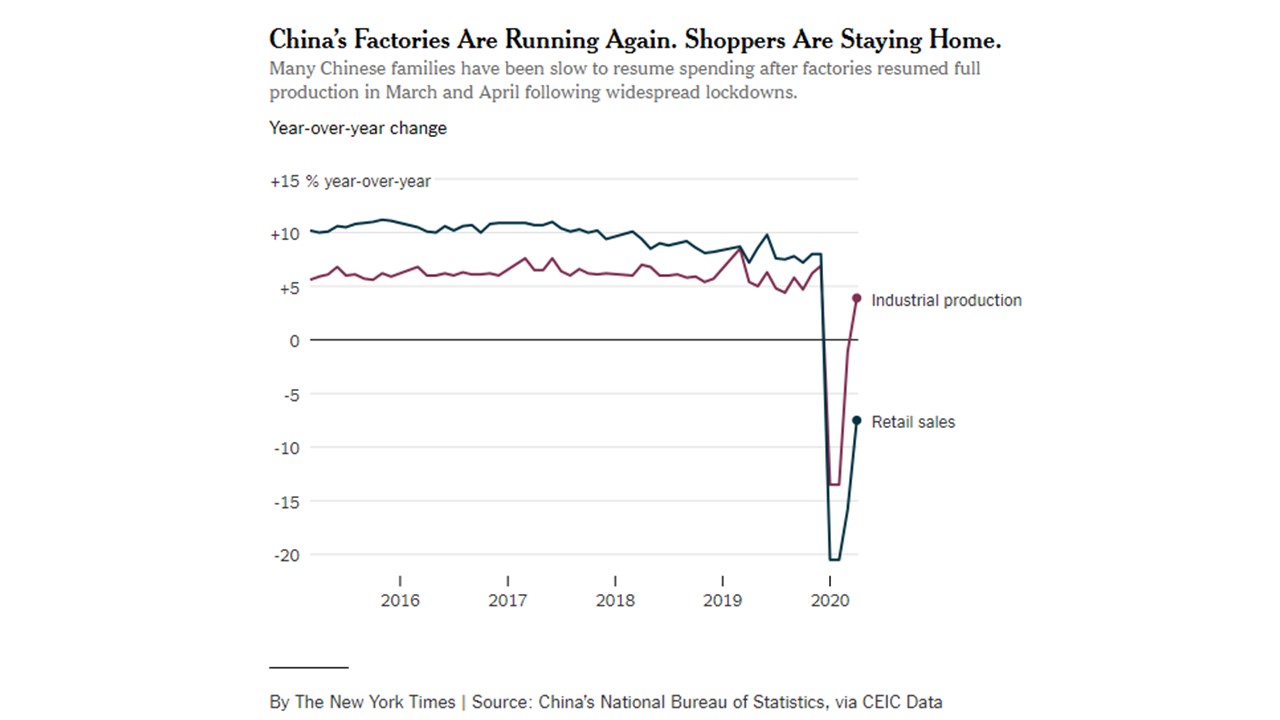
The world is watching China’s economic performance closely. It is a couple of months ahead of the rest of the world in coping with the virus and then trying to reopen businesses. Its successes or stumbles could offer lessons to others…
“We should be aware that given the continuous spread of the epidemic abroad, the stability and recovery of the national economy is still faced with multiple challenges,” Ms. Liu said.
The difficulties are prompting more and more warnings that China – and possibly other countries after it – may face a “W-shaped” pattern of economic activity.
The National People’s Congress is scheduled to convene for its annual session starting next Friday, 11 weeks after it is usually held. Beijing also announced on Friday that it would ease restrictions on banking and other financial services among Hong Kong and adjacent areas of mainland China in an attempt to increase economic growth there.
In such a pattern, the economy nose-dives when most businesses close during lockdowns and then seems to recover when factories and stores reopen. But with many consumers still scared of infection and leery of spending money, the economy then dips a second time before embarking on a more sustainable recovery.
As the world’s largest oil importer by a wide margin, China is also enjoying a huge windfall from the plunge in global oil prices. “China’s growth this year could surprise on the upside,” Mr. Xu said.
- 05/18/2020 – Six High Frequency Indicators for the Eventual Recovery from calculated risk
by Calculated Risk on 5/18/2020 08:16:00 AM. These indicators are for travel and entertainment – some of the sectors that will probably recover very slowly.
The TSA is providing daily travel numbers.Click on graph for larger image.
This data shows the daily total traveler throughput from the TSA for 2019 (Blue) and 2020 (Red).
On May 17th there were 253,807 travelers compared to 2,620,276 a year ago.
That is a decline of 90.3%. There has been some increase off the bottom, but it is pretty small compared to the normal level of travel.
The second graph shows the year-over-year change in diners as tabulated by OpenTable for the US and several selected cities.
Thanks to OpenTable for providing this restaurant data:
This data is updated through May 16, 2020.
The US was off 100% YoY as of March 21st.
California and New York are still off 100%.
Some states – like Texas and Georgia – have started to open up. In Texas, diner traffic was only down 75% YoY.
This data shows domestic box office for each week (red) and the maximum and minimum for the previous four years. Data is from BoxOfficeMojo.
Note that the data is noisy and depends on when blockbusters are released.
Movie ticket sales have been essentially at zero for eight weeks.
Basically movie theaters are closed all across the country, and will probably reopen slowly (probably with limited seating at first).
The following graph shows the seasonal pattern for the hotel occupancy rate using the four week average.
The red line is for 2020, dash light blue is 2019, blue is the median, and black is for 2009 (the worst year probably since the Great Depression for hotels).
2020 was off to a solid start, however, COVID-19 has crushed hotel occupancy.
Notes: Y-axis doesn’t start at zero to better show the seasonal change. I added 2001 to show the impact on hotel occupancy after 9/11.
STR reported hotel occupancy was off 55.9% year-over-year last week. Occupancy has increased slightly over the last few of weeks.
This graph, based on weekly data from the U.S. Energy Information Administration (EIA), shows the year-over-year change in gasoline consumption.
At one point, gasoline consumption was off almost 50% YoY.
As of May 8th, gasoline consumption was off about 20% YoY (about 80% of normal).
The final graph is from Apple mobility.
This data is through May 16th for the United States.
According to the Apple data, driving is back to normal, walking is a somewhat below normal, but public transit is still off 69% from the pre-crisis level.
- 05/17/2020 – Powell seems to be quite confident with recovery of US economy and thinks it will grow in the second half of 2020
Federal Reserve Chairman Jerome Powell on the coronavirus-ravaged economy
The head of the U.S. central banking system tells Scott Pelley how high he thinks unemployment will go, what tools the Fed still has to breathe life into the economy and what outcomes he’s trying to avoid on the road to economic recovery.
Federal Reserve Chairman Jerome Powell urged the White House and Congress to spend more money to ensure their initial response to the coronavirus-induced economic contraction isn’t wasted amid evidence that recovery will take longer than first thought.
full transcript, except here
POWELL: So the level of economic activity is going to decline substantially in the second quarter. It will be reported at an annual rate, which will basically be four times as much as the amount it shrank in that quarter. But it’s going to be a sharp decline by any measure. And, really, without precedent. And let’s remember though that this is something that we’re doing as a society, really, to protect ourselves from the virus. This is not because there was some inherent problem, a housing bubble or something like that or the financial system in trouble. Nothing like that.
The economy was fine. The financial system was fine. We’re doing this to protect ourselves from the virus. And that means that when we do that, when the virus outbreak is behind us, the economy should be able to recover substantially. And I expect that it will recover substantially, but that it will take some time.
- 05/17/2020 – the Market Rally might be a prolonged process due to uncertainty
Bets on Slow Economic Recovery Challenge Market Rally
Investors increasingly believe that fallout from the coronavirus pandemic will last longer than they initially thought
The recent market rally is uneven, focused more on big tech companies, industrials and financials are left out.
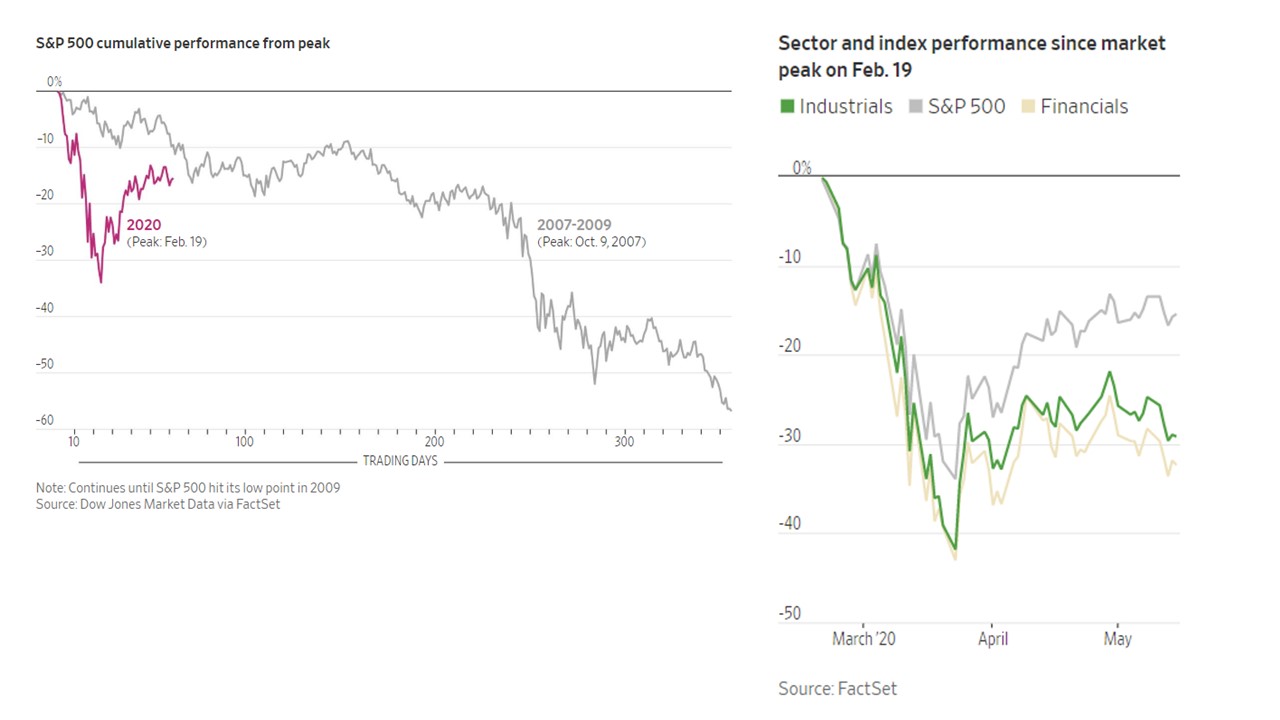
Earnings estimates for the full year have tumbled across sectors, but analysts say a wide range of possible outcomes are making such projections hard to interpret.
In April, an index of global economic-policy uncertainty developed by professors at Northwestern University, Stanford University and the University of Chicago rose to its highest level on record in data going back to 1997.
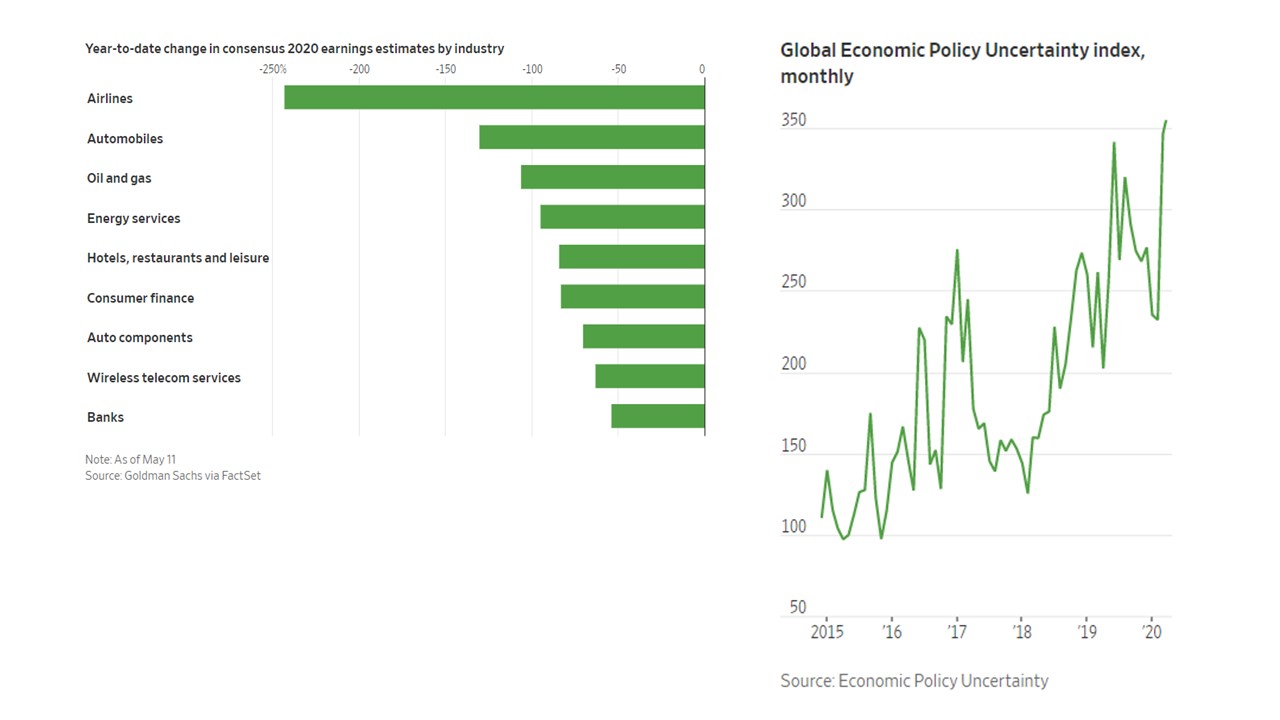
- 05/16/2020 – Trump tries to work with GOP to pass part of phase 2 stimulus plan
Trump to Meet With GOP Lawmakers as U.S. Coronavirus Death Toll Tops 88,000
Camp David gathering comes as president indicates he hasn’t ruled out new aid to states, municipalities
The House package, which passed 208 to 199 with 14 House Democrats voting against it, includes about $1 trillion in direct aid to states and localities to deal with the effects of the pandemic.
Republicans made clear that the bill has no prospect of advancing in the GOP-led Senate, but President Trump hasn’t ruled out additional economic relief for states and cities grappling with the pandemic, as long as it doesn’t constitute what he considers a bailout, White House economic adviser Kevin Hassett said Friday. Mr. Trump was expected to meet with House Republicans at the president’s Camp David retreat this weekend.
- 05/16/2020 – economy contraction maybe bottom out and shows sign of recovery
After Devastating Economic Contraction, Glimmers of Growth Emerge
Daily and weekly data suggest a recovery might be brewing, though its strength is unclear
map requests on Apple Inc. devices fell 50% throughout the country between mid-January and the week ended April 9, but they have steadily climbed since then and are now down just 20%. retail visits show the same trend, according to Unacast, a mobility-data analytics company: off more than 50% in mid-April from a year earlier, but down just 32% this past week. Real-estate brokerage Redfin Corp. said home-buyer demand as measured by customers contacting affiliated agents, after plummeting by one-third, is now above prepandemic levels.
- 05/15/2020 – House Dam starts the negotiation of Phase 2 stimulus plan with GOP. Good sign if part of it gets pass.
House Narrowly Passes $3 Trillion Aid Package
The House narrowly passed a sprawling, $3 trillion coronavirus-relief package Friday night, capping a weeklong effort by Democratic leaders to quash rebellions from various wings of their party.
The House bill includes about $1 trillion in direct aid to states and localities, including grants and education assistance, to deal with the effects of the pandemic. It would put a new round of one-time cash payments into Americans’ bank accounts, extend the duration of enhanced jobless benefits, help cover some rents and mortgages, forgive some student-loan debt and send premium pay to essential workers in fields such as health care.
Republicans made clear that the bill has no prospect of advancing in the GOP-led Senate. “It’s a parade of absurdities that can hardly be taken seriously,” Senate Majority Leader Mitch McConnell (R., Ky.) said Thursday on Fox News. Mr. McConnell said that he had spoken recently with President Trump and cabinet officials and that they agree another bill is probably necessary but that “it’s not going to be a $3 trillion left-wing wish list like the speaker is apparently going to try to jam down the throats of her majority.”
Mr. McConnell has said the next bill must include measures to shield companies from liability during the pandemic.
White House economic adviser Kevin Hassett said in an interview Friday with The Wall Street Journal that Mr. Trump is open to more funding for state and local governments, as long as the money isn’t used to “bail out states that haven’t necessarily had their act together.” Congressional Republicans have said they don’t want to allow states to use the federal funds to support pension programs.
- 05/15/2020 – Good news from China on refinery picks up. Does this cause the early 33% pop of CHK stock?
China crude oil runs rebound in April as fuel demand picks up
* April crude runs 13.1 mln bpd, 11% higher on month
* Fuel demand recovers as coronavirus lockdown unwinds
* Jan-April refinery runs down 3.4% on yr
* April natural gas output +14% on yr; crude oil +0.9%
BEIJING/SINGAPORE, May 15 (Reuters) – China’s daily crude oil throughput rebounded in April from a 15-month low in March as refiners cranked up operations to meet renewed fuel demand after lockdowns imposed to prevent the spread of the coronavirus outbreak were eased.
The country processed a total of 53.85 million tonnes of crude oil last month, data from the National Bureau of Statistics (NBS) showed on Friday, equivalent to about 13.1 million barrels per day (bpd).
That some 11% higher than 11.78 million bpd in March, and also 0.8% above the level for April 2019.
- 05/14/2020 – potential trade war might derail the economy recovery path. I need to watch out on this and hedge my positions
- From the U.S. to Europe to Australia, more and more world leaders are calling for China to be investigated over the origins of the outbreak, which was first reported in the Chinese city of Wuhan in late December.
- Escalating tensions over China’s handling of the global pandemic pose a “major risk” to economic recovery and could lead to a trade war worse than the one between Beijing and Washington, David Sokulsky of Concentrated Leaders Fund said.
- Sokulsky said the situation may worsen further: “We could see a very quick rehashing of the trade wars that we had, but potentially on a much worse scale than what we had last year.”
- 05/14/2020 – here is med knowledge of general CV. CV-19 may live with us forever and there might never be vaccine for it like other CVs.
- 05/14/2020 – the global debt issue is looming, need to review in details (think about 2014 global debt crisis)
Coronavirus to leave a legacy of unprecedented global debt
- 05/14/2020 – I should be very cautious going into Winter, there might be 50% chance the 2nd wave will be back if US does not have a good mitigation plan
U.S. faces ‘darkest winter’ if pandemic planning falls short: whistleblower
- 05/13/2020 – his comments are quite sober and positive, lay the path for further stimulus. Should not be a negative sign for the market
An Economic Update by Fed Chair Jerome H. Powell | Event | PIIE – video
Stocks Fall About 2% as Powell Says Outlook ‘Highly Uncertain’ – wsj news
Worries about fresh outbreaks of coronavirus as well as rising U.S.-China-tensions dampen sentiment
highlights of Powell’s comments,
- Congress has provided roughly 2.9 trillion dollars in support for households businesses Healthcare Providers and state and local governments about 14% of GDP. the Fed measures taken so far to offset the economic effects of the virus and the path ahead governments around the world have responded Swift and forcefully.
- Fed acted with unprecedented speed and force after rapidly cutting the federal funds rate close to zero. And has a wide array of additional measures to facilitate the flow of credit in the economy which can be grouped into four areas: 1) outright purchases of treasuries and agency mortgage-backed Securities to restore functionality and the’s critical markets liquidity; (2) funding measures including discount window measures expanded swap lines with foreign central banks and several facilities with treasury backing to support smooth functioning in money markets; (3) additional backing from the treasury facilities two more direct directly support the flow of credit to households businesses and state and local governments; (4) temporary regulatory adjustment to encourage and allow Banks to expand their balance sheets to support their household and business customers. Quite a few evidence show that our actions work.
- He revealed growing alarm about the path ahead, which the Fed leader described as “highly uncertain and subject to significant downside risks. Additional fiscal support could be costly but worth it if it helps avoid long-term economic damage and leaves us with a stronger recovery,”
- The Fed is already purchasing Treasury and mortgage-backed securities and has ballooned its balance sheet to $6.7 trillion with aggressive purchases.
- Powell did say other policy moves may be necessary, and the economy may need more help through fiscal stimulus, which would have to be authorized by Congress. “Additional fiscal support could be costly, but worth it if it helps avoid long-term economic damage and leaves us with a stronger recovery,” Powell said.
- Powell’s description of the economy was bleak, saying it caused “a level of pain that is hard to capture in words.” Powell also said a recovery is dependent on the virus, how long it will take to develop treatments and whether the economy can rebound despite the limitations of social distancing. It will also depend on whether there are new outbreaks.
- Fed would NOT use negative rates to stimulate the economy because its effect is mixed.
- Long term unemployment is not for the economy. The unemployment might peak next month and will gradually go down over the months or years. But it is a long term recovery. Too bad, we had the smallest unemployment rate in 50 years and it all gone in two months.
General knowledge: what the Fed Reserve can do to fight recession? they want to make sure we do not have a financial crisis.
- Monetary policy, interest rates
- lender of last resort: government bonds, repurchase market cash injection, discount window lending, commercial papers (used for day to day payroll), swap lines for international market
05/13/2020 – If Trump sanctions China, market will probably slump significantly. But on the other hand, US might pump more liquidity and start infrastructure plan to stimulate the economy internally.
GOP senators introduce bill permitting Trump to sanction China over failure to cooperate on COVID-19
- 05/13/2020 – David Tepper said market is overvalued by most standards, but because Fed puts lots of liquidity, market can still can go up. The risk/reward is high. So be very cautious.
David Tepper says this is the second-most overvalued stock market he’s ever seen, behind only ’99
- 05/12/2020 – it might take a while for this bill to get legislated and in the end, the final bill should be a very compromised bipartisan one. But anyway, another stimulus plan might be on the horizon in the late May or early June or whole June.
House Democrats Release $3 Trillion Bill to Respond to Coronavirus
Bill allots $1 trillion for states and local governments, including funds for education, public safety; sum is roughly double Congress’s pandemic spending so far
Democrats acknowledge that the bill introduced on Tuesday is an opening bid. “The larger bill is a negotiating start point,” said Rep. Stephanie Murphy (D., Fla.) in a call with reporters. “I really do believe in a divided Congress, the bills that have bipartisan support, that have the ability to have significant impact on the lives of Americans who are suffering right now, are the things that will eventually make it into law.”
Formal Talks Paused on Next Stimulus Package, Trump Economic Adviser Larry Kudlow Says
White House press secretary Kayleigh McEnany said Mr. Trump was looking forward to talks.
“I think it’s important for us to move and look at a Phase 4,” she said, referring to the next round. “The president thinks so too. So those negotiations will happen.”
- 05/12/2020 – here are my takeaways from the following testimonies (including Dr. Fauci)
- if we reopen too fast and do not follow the guideline, we might have great risk of cases rebound
- there is real possibility that we will have vaccine by the end of this year because majority of people do not have the symptom which means that we can develop antibody
- all the predictions can be wrong since we do not know comprehensively of this virus
- Senator from South Carolina said we have successfully flatten the curve and the previous model prediction was way off. Dr. Fauci agreed.
- lots of people probably already have immunity of this virus
- Dr. Fauci is just health and medical advisor, not policy maker. His standpoint is only scientific based on evidence
Top health officials testify before Senate on safe reopening amid Covid-19 – 5/12/2020
- Fauci, CDC’s Redfield and other top health officials testify before Senate committee on reopening US economy
- The U.S. Senate committee on Health, Education, Labor and Pensions is holding a hearing Thursday to question top officials from the White House Coronavirus Task Force about plans to ease restrictions and reopen the economy.
- Director of the National Institute of Allergy and Infectious Diseases Dr. Anthony Fauci is scheduled to speak as well as Dr. Robert Redfield, director of the Centers for Disease Control and Prevention.
- Assistant Secretary for Health admiral Brett Giroir and Stephen Hahn, Commissioner of the Food and Drug Administration, are also due to speak.
Fauci reportedly plans to warn states that prematurely reopening their economies will cause “needless suffering and death,” according to The New York Times.
“The major message that I wish to convey to the Senate HLP committee tomorrow is the danger of trying to open the country prematurely,” Fauci wrote to the Times. “If we skip over the checkpoints in the guidelines to: ‘Open America Again,’ then we risk the danger of multiple outbreaks throughout the country. This will not only result in needless suffering and death, but would actually set us back on our quest to return to normal.”
President Donald Trump issued broad federal guidelines about a month ago titled “Opening Up America Again.” The guidance lays out criteria for states that includes 14 days of decreases in daily new cases among other measures. States, which have imposed their own containment measures to try to slow the spread of the disease, are not legally required to follow the White House’s instructions.
Dozens of states have since reopened parts of the economy and lifted restrictions. Some states, such as Georgia, did so without meeting the criteria laid out by the White House. The CDC has since created a 17-page report titled “Guidance for Implementing the Opening Up America Again Framework,” which was researched and written to help faith leaders, business owners, educators and state and local officials as they begin to reopen.
The report was supposed to be published at the beginning of this month, but was shelved by the Trump administration. Agency scientists were told the guidance “would never see the light of day,” according to a CDC official.
- 05/11/2020 – will we see the second wave come back? I need to be very cautious.
- 05/10/2020 – history of pandemic 1918 – three waves: April 1917, Sept 1918, Jan 1919
1918 Pandemic Influenza Historic Timeline
How the Horrific 1918 Flu Spread Across America
The toll of history’s worst epidemic surpasses all the military deaths in World War I and World War II combined. And it may have begun in the United States
- 05/09/2020 – oil storage in close to full: Cushing 70 percent (53/76 mil), reserve is 89% full. This might drop the oil price again if demand does not pick up soon.
U.S. Oil Storage May Have Room For ‘’Several Hundred Million Barrels’’
The Strategic Petroleum Reserve could hold up to 714 million barrels of crude. Currently, its occupancy is about 636 million barrels, Reuters notes.
The Cushing, Oklahoma, hub is also filling up. The current occupancy rate of the complex is about 70 percent, but according to traders, it is actually fully booked by companies seeking storage space for their oil. Cushing’s maximum capacity is about 80 million barrels, but its working capacity is 76 million barrels, according to Reuters. As of April 17, the amount of oil stored there stood at 53 million barrels.
Oil is being stored offshore as well. Off the coast of California alone, there are tankers loaded with some 20 million barrels of crude, idled offshore with the oil nowhere to go.
- 05/09/2020 – Given the daily rate of increase of TSA (about 20%), it will take 14 days to reach normal TTT (total traveler throughout) (215,444*1.2^14 =2,766,125 ~ 2,602,631). Oil demand might increase in 2 weeks.
TSA checkpoint travel numbers for 2020 and 2019
- 05/03/2020 – BH annual shearholders meeting 2020
Warren Buffett and the 2020 Berkshire Hathaway Annual Shareholders Meeting [FULL EVENT]
- 04/28/2020 – banks are all selfish, so financial system will be back sooner than small businesses?
joshua rosner @JoshRosner Replying to @CNBC
Everyone’s missing the key to the #PPP story. It’s not that #TBTF banks are giving loans to public companies. I’ve analyzed the filings & found they are FIRST providing
@SBAgov funds to Co’s to which the banks have direct prior debt exposures. Self bailout
@federalreserve @USOCC
- 04/27/2020 – I also can use TSA website to track the daily travel and see when it is back to normal.
Good article from calculated risk blog: The TSA is providing daily travel numbers.
This is another measure that will be useful to track when the economy starts to reopen.
Click on graph for larger image.
This data shows the daily total traveler throughput from the TSA for 2019 (Blue) and 2020 (Red).
On April 26th there were 128,875 travelers compared to 2,506,809 a year ago.
That is a decline of 95%.
- 04/27/2020 – great comments on current situation from Scott Grannis. It is time to all in?
The Covid-19 crisis is not over yet, but the light at the end of the tunnel is getting much brighter. Key financial indicators are moving in a healthy direction, and most countries are seeing clear signs that the viral outbreak is under control.
More and more analysts are finding that shutdowns haven’t resulted in better results than non-shutdowns. Sweden is the perfect example.
21 states now meet the federal “reopening” criterion of a 14-day downward trajectory of daily new cases. 22 states meet the criterion of a 14-day downward trajectory in the percentage of tests with positive results. California already meets the second, but not the first. New York (yes, NY!) meets both.
The initial predictions of deaths and hospital over-crowding were so far off the mark (i.e., way too high) as to be almost criminal. The biggest problem most hospitals face today is bankruptcy because so many beds are empty. The mandate that hospitals should accept only covid patients was also criminal. Furthermore, the projected shortage of ventilators—a major factor driving the decision to shut down the economy in order to “flatten the curve”—was a criminal distraction, because it is now clear that curves have flattened everywhere and ventilators are only marginally helpful in preventing deaths. New York is now giving away tens of thousands of ventilators that were never used.
It is now painfully obvious that “The shutdown of the US economy will prove to be the most expensive self-inflicted injury in the history of mankind.™”
It should also be painfully obvious that we should reopen economies asap.
- 04/26/2020 – Mnuchin is confident that economy will bounce back quickly in the third quarter since it is not a financial problem but government shut the economy down
Mnuchin Sees Third-Quarter Rebound for U.S. Economy (fox video)
- 04/23/2020 – need to fully understand this
USO Now Detaching From Reality
- 04/23/2020 – USO is in bad contango. If the inventories continue to build up through mid-May, both speculative investors and the United States Oil fund will once again dump their June contracts, leading to another massive decline in WTI crude oil prices.
The US Oil ETF Is Not the Best Vehicle to Bet on Oil
How did oil fall into negative territory?
- According to data from Bloomberg, the United States Oil fund owned approximately 25% of all outstanding May contracts, and the fund dumped these in the open market to buy June contracts. This created a massive sell-side pressure.
- Speculative traders who never had the intention of owning physical oil barrels followed suit and disposed of their positions.
- Generally, commercial buyers come to the rescue and purchase these contracts. These are the companies that store oil for business purposes. However, this time around, the storage capacities were already approaching a peak due to the reduced demand for oil in the last few weeks while supply remained at elevated levels. There was no demand for the expiring contracts even though the price fell to record lows.
The combination of these three developments led oil into negative territory last Monday. The Brent crude, on the other hand, did not follow suit, as storage capacities did not become a question for the European benchmark. Even more important to note is the fact that June contracts are still trading above $14 per barrel.
Undermining the fundamental problem of declining demand for oil as a result of the global lockdown is not wise, but this was not the driver of oil into negative prices. The futures pricing mechanism and the shortage in storage facilities are to be blamed for this historic drop
More short-term troubles for energy investors
The storage problems in the United States might continue in the next month as well. This is bad news for the industry. The supply cuts proposed by the OPEC+ alliance are welcome news, but it would take a few weeks for stored oil to reach the market. Therefore, Cushing oil storage facilities will most likely operate at unusually high utilization levels.

Source: S&P 500 Global Intelligence
If the inventories continue to build up through mid-May, both speculative investors and the United States Oil fund will once again dump their June contracts, leading to another massive decline in WTI crude oil prices.
From a more fundamental perspective, the demand for the commodity is not expected to stage a comeback in the next month as well, primarily due to the global lockdown that has brought manufacturing activities to a standstill.
Contrary to the belief of many retail investors, the fund does not directly invest in oil, and neither does it take physical delivery. Rather, the fund buys front-month contracts and then rolls over on to the next month’s futures before the expiry of these financial instruments. In every sense, the United States Oil fund should be treated as a derivative, not a direct investment in the commodity. Therefore, an investor would be exposed to a few additional risks when using this fund to gain exposure to the energy market, as opposed to betting on the equity securities of publicly listed energy companies such as Exxon Mobil Corporation (NYSE:XOM).
There’s another important development that has made investing in this fund a very risky way to bet on the expected recovery of energy prices. The oil market is in contango, meaning that the futures contracts are trading at a premium to the spot price, which is an ominous sign for the fund. According to data from the U.S. Energy Information Administration, the futures price is $10.01 per barrel in comparison to the spot price of $8.91 on April 22. As long as this phenomenon continues, the fund will be making losses during the process of rolling over the contracts, as this is equivalent to selling low and buying high.
- 04/23/2020 – USO does not track spot price any more due to its structure change?
Young investors rush into struggling oil ETF that isn’t even tracking the price of oil anymore
The fund began to run into trouble when the May contract for oil began diving last week. This week, the May contract fell to an actual negative price. The fund likely had already sold those contracts for the June oil futures, but the fall to negative prices sent a chill through the whole oil market. June oil futures then began plummeting this week as well and traders feared they would turn negative.
This caused the fund to implement a series of changes beginning last Friday. The fund, originally set up to invest in just the front-month contract until two weeks before expiration, said it would now invest funds in futures expiring months out. It also suspended the making of creation baskets, which is how an ETF makes new shares to meet demand. The creation baskets buy the futures. But by suspending this mechanism this week, the fund began to trade as a closed-end fund with a fixed number of shares. This also meant it could no longer accurately track the price of oil.
Retail investors buying the fund likely aren’t aware that it is no longer tracking oil prices in lockstep.
For example on Wednesday, the fund dropped 11% even as oil’s June futures jumped 20%. On Thursday, while June oil jumped 30%, the USO only gained 12%.
When asked why the fund keeps changing its structure, the company’s chief marketing officer told CNBC the following: “Due to extraordinary market conditions in the crude oil markets, including super contango, USO has invested in other permitted investments, as described in the prospectus.”
- 04/23/2020 – are there any investment opportunities here?
- hedge funds shorts USO and made a lot of money from retail investors because Retail has been plowing into these oil contracts thinking they’re buying spot crude oil when they’re buying the next front month. So they’re paying $22 a barrel when the spot market’s negative $38.
- Good to know the fund is going to executed an 8-for-1 reverse stock split and change its structure to hold a mixture of contracts instead of front month contract. Need to check the details in its prospectus to understand whether it is investable or not.
- From Kyle Bass: the last trading day of USO May contract is 04/20, June contract is May 19. The price crash is due to lack of storage not because of spot price. The long term contract still price as $31. It might take 60 days to reopen econ. Sardi and Russian declared oil war to US in order to destroy shale oil which happened at the exact time of CV crisis. Sardi’s balance sheet can sustain years , Russian is using currency to overcome the price drop. US should be able to deal with this due to superior management structures. SP500 is overpriced now. Hope government will do more to boost econ.
Short sellers make nearly $300 million betting against retail investors’ favorite oil fund
Hayman Capital Management CIO Kyle Bass has been warning investors about the danger of exchange traded funds that track oil prices and said he was short some of these funds.
“Retail has been plowing into these oil contracts thinking they’re buying spot crude oil when they’re buying the next front month. So they’re paying $22 a barrel when the spot market’s negative $38. Retail investors are going to get fleeced if they continue to fly into these oil ETFs,” he said Monday on CNBC’s “Closing Bell.”
- 04/22/2020 – Dem and WH have bigger 4th CV relief plan (including infrastructure plan), but Rep casts doubts and wants to discuss about national debt first. Senate is scheduled to be back in May 4. Uncertainty will come by then.
Here are Congress’ next steps in response to coronavirus pandemic
- Democrats and the White House already have a list of priorities for the next congressional coronavirus relief plan.
- But Senate Majority Leader Mitch McConnell cast doubts about spending more taxpayer money to respond to the pandemic.
- The Senate passed a $484 billion relief package Tuesday, and the House is set to approve it Thursday.
“There will be a big, broad, bold [fourth relief bill]. For anyone who thinks this is the last train out of the station, that is not close to the case,” Senate Minority Leader Chuck Schumer said at a news conference Tuesday with his fellow Democratic leader House Speaker Nancy Pelosi after the Senate passed the $484 billion legislation.
In a pair of tweets Tuesday, Trump called to pass more money for state and local governments stretched to their limits. He pushed for an infrastructure package, complete with money for roads, bridges, airports and rural broadband — which Democrats support. The president also called for tax incentives for sports and entertainment businesses, along with a payroll tax cut.
“After I sign this Bill, we will begin discussions on the next Legislative Initiative with fiscal relief … to State/Local Governments for lost revenues from COVID 19, much needed Infrastructure Investments for Bridges, Tunnels, Broadband, Tax Incentives for Restaurants, Entertainment, Sports, and Payroll Tax Cuts to increase Economic Growth,” Trump wrote.
But hesitation from Republican lawmakers to add to historic emergency spending could trip up any ambitions Democrats and the White House have. After the Senate passed the bill Tuesday by unanimous consent with only a few lawmakers present, Majority Leader Mitch McConnell cast doubts about moving quickly on another relief package.
“But my view is we’ve gone so far on the national debt here that the next time we address this issue, the Senate should be back in session,” the Kentucky Republican said. “Fully up and running with everybody involved in discussion. So I will predict that we will not try to pass another rescue package by consent. My view is we ought to bring everybody back, have full participation to begin to think about the implications to the country’s future for this level of national debt.”
The full Senate is expected to return to Washington on May 4.
Speaking to radio host Hugh Hewitt on Wednesday morning, McConnell said he does not back using more federal money to buoy state governments. He indicated he would support allowing states to declare bankruptcy.
- 04/22/2020 -Mnuchin wants more relief packages, McConnell wants to pause.
Mnuchin Says ‘We Need to Spend What It Takes’ to Overcome Coronavirus Crisis
- 03/26/2020 – COVID-19 Q&A with Dr. Fauci and Stephen Curry (Official Video)
- 03/19/2020 – Simulation of CV
Why outbreaks like coronavirus spread exponentially, and how to “flatten the curve”
- 03/07/2020 – Comments from experts of Harvard University
At Harvard forum, three who know warn of ‘most daunting virus’ in half a century
哈佛会议实录 | 新冠肺炎的全球影响,听听世界顶级学者怎么看
- 03/07/2020 – Gilead’s antiviral drug Remdesivir might get phase 3 results before end of April
A closer look at the Ebola drug that’s become the top hope for a coronavirus treatment
“There’s only one drug right now that we think may have real efficacy. And that’s remdesivir.” said Bruce Aylward, a senior advisor and international leader of the World Health Organization’s joint mission to China, at a Feb. 24 press conference.
Clinical trials are now underway in the U.S., China and, soon, other Asian countries where high numbers of people are infected by the virus, now named SARS-CoV-2. Preliminary results from the first of those studies could come by April, Gilead expects.
Other drugs designed specifically to treat SARS-CoV-2 are now in development but, for the next few months at least, much of the focus will be on remdesivir. Here’s a primer on how it works, its potential against the illness COVID-19, as well as why it’s no sure thing.
What kind of drug is remdesivir?
Remdesivir is designed to slow the infection of healthy cells by blocking viral replication, checking the ability of an invading virus to co-opt the body’s cellular machinery to reproduce itself.
In technical terms, it’s known as a nucleotide analog — meaning it mimics a genetic building block, but with an unusual molecular group tacked on. The thinking goes that, as the invading virus tries to replicate itself, the drug gets in the way and stops the process.
Remdesivir emerged from work done by Gilead and U.S. government in the mid-2010s to test compounds discovered by the biotech against emerging viruses. Initial animal experiments seemed to show the drug could work against Ebola, leading Gilead to start Phase 1 testing in 2015 and advance it into a mid-stage trial the next year.
A large Phase 3 study conducted in the Democratic Republic of the Congo, however, showed remdesivir to be less effective in preventing deaths from the virus than two other drugs, leaving Gilead’s therapy with an uncertain future.
But the outbreak of SARS-CoV-2 made suddenly relevant a series of experiments in human cell cultures and mice that indicated remdesivir could have activity against coronaviruses like MERS and, possibly, the new one now spreading.
What evidence suggests remdesivir could treat COVID-19?
Gilead has taken pains to emphasize, when announcing new plans for remdesivir, that it lacks human data on whether the drug actually works to treat COVID-19.
What it does have is preclinical data showing the drug to be active against the MERS and SARS viruses, which share enough genetic similarities to SARS-COV-2 that researchers and the company think remdesvir could work against it too.
Testing by National Institutes of Health researchers, published in January, demonstrated remdesivir prevented MERS from developing in monkeys, and helped improve the condition of those animals that were already infected.
“Our results, together with replication inhibition by remdesivir of a wide range of coronaviruses in vitro and in vivo, may further indicate utility of remdesivir against the novel coronavirus […] emerging from Wuhan, China,” they wrote.
Most recently, an in vitro study conducted by scientists at the Wuhan Institute of Virology found remdesivir and an anti-malarial called chloroquine more likely to be effective against SARS-CoV-2 than a handful of other potential treatments.
As remdesivir has drawn attention, many have focused on its use in treating a Washington man who tested positive for SARS-CoV-2 after returning from Wuhan. One week into his hospitalization, doctors gave him remdesivir through a compassionate use program that Gilead has been supporting.
The man’s condition began to improve the next day, sparking some optimism about remdesivir’s benefit. But it’s not clear if the patient had already begun to recover, as viral levels were declining before the drug’s use.
How is remdesivir being tested now?
Early last month, doctors in Wuhan and Beijing opened two Phase 3 studies of remdesivir in coronavirus-infected patients with either mild to moderate illness or with severe disease. The former began enrolling on Feb. 6 and the latter a week later, on Feb. 13.
Together the trials aim to treat about 850 people and measure whether intravenous remdesivir can improve or resolve the symptoms of COVID-19 over the course of roughly a month.
another relevant Chinese news 瑞德西韦最新消息
在疫情的胶着期,随着磷酸氯喹都进入了新冠肺炎治疗的最新方案,对于早已被给予极大关注的瑞德西韦的临床实验进展情况,人们更是翘首以盼。可惜这方面的信息真的是少之又少,各个方面都是慎而又慎,毕竟磷酸氯喹、柯立芝等是老药新用,在疫情的时间轴上占尽了优势,而中草药更是在试验的方面有着体制性优势。但是无论如何,作为一种已经申请了针对冠状病毒有效专利的新药,瑞德西韦确实承载了人民的希望,而且随着疫情在全球的肆虐,这个人民已经演变成了全球人民的希望。
为此,如果能听到瑞德西韦研究主持人曹彬教授针对瑞德西韦在临床试验进展方面的直接回应,将能够极大的解开瑞德西韦到底行还是不行的迷雾!以下是有关采访实录,以飨读者:
采访:我们谈一谈大家非常关注的「瑞德西韦」这个药。
曹彬:瑞德西韦这个药物进入我们的视野是在洛匹拉韦/利托那韦(柯立芝)之后,也就是在2020年1月9日,我就把我们的第2个目标锁定到瑞德西韦。
就在这个研究当中,我们惊讶的看到,MERS冠状病毒的动物模型中,洛匹拉韦/利托那韦+干扰素能够保护小鼠、减少肺损伤,降低小鼠的死亡率,但病毒的下降幅度,并没有差别。我们又看到瑞德西韦同样能够保护小鼠、同样能降低肺的损伤,同样能降低小鼠的病死率。而且,瑞德西韦降低病毒滴度的能力是很显著的。这给我们一个非常大的震撼,洛匹拉韦/利托那韦+干扰素方案的效果远远不如瑞德西韦,我们也很幸运的发现,这个药物原来开展了人体研究。
这项研究给我们信心,就是说,瑞德西韦这个药物至少已经在非洲人身上进行过人体试验了,我们能看到在非洲人群的药物安全性数据。
采访:我能不能这样理解,一个药物有没有效果和安全性,两项重要的指标,在「有没有效果」方面,和「安全性」方面。
曹彬:对,所以说非常幸运的,我们既拿到了动物实验有效性的证据,又拿到了在人体试验当中安全性的数据。所以我们立刻主动联系开展瑞德西韦试验。
采访:大家比较关心的问题,也就是「结局」,试验终点,是出于什么考虑?
曹彬:因为轻症病人大多是自限的,所以我们在对轻中症患者的设计方面,我们和重症患者设计是完全不一样的。实际上这是两个完全不同的临床研究,对于重症新冠肺炎,我们关心的它的「硬终点」,就是说它能够导致患者致死、致残的这样终点,当然死亡是我们硬终点之一,但不是全部。
对于轻症病人来说,我们当然不排除有极少数的轻症病人最后转成重症,这是有可能的,但从这个疾病的规律来说,85%以上的病人是自限性的疾病。
采访:我相信大家能够理解,我们这样的研究还是积累的结果,是来自于之前大量临床研究和文献阅读的总结和设计,也希望大家能够理解我们这种临床结局的设定。
曹彬:咱们瑞德西韦2的研究当中,有中期分析的研究设计,中期分析不是由研究者分析的,它有一个独立的安全委员会来进行分析的,我们瑞德西韦临床研究的独立委员会共有5个人,其中两位是国内专家,三位是国外专家,包括美国一位、加拿大一位、英国一位,这五位专家中有三位是统计学方面的,另两位是临床专家,他们从后台看我们的数据,然后去评判。而且还不仅仅是中期,他们可以有计划地查看项目进展、实时的去看两组之间的疗效差异,虽然实际上是随机的安慰剂对照双盲的,但是它的效果有可能会呈现出「离散度越来越大」的态势,如果药是有效的,那么离散度会越来越显现出来。
采访:我能不能理解成,要么是特别有效,要么是危害性特别大?
曹彬:可能有三种可能,一是:死亡率增加;二是病死率下降。第三种可能性是:除了有效和有害,还有活性药物在标准治疗上无额外作用的可能性。
当达到一个节点的时候,独立委员会可能会叫停这个试验。如果是活性药,证实有效的话,这个试验就停止了,所有的病人就都建议使用活性药物。如果是另外一种情况,副作用特别大,也必须叫停实验,这个药就被「枪毙」掉了,以后再也不允许这个药物在人体当中开展临床研究。
采访:虽然我们目前不太了解最终的结果,那能不能有一些能够「透露」给我们的信息?
曹彬:我们现在瑞德西韦2的研究已经超过了230例,已经达到了中期分析所需要的样本量,但是「达到样本量」和「能进行中期分析」是完全两个不同概念意义,为什么呢?因为入组不代表就可以进行评价了,还需要28天的随访。
我还想解释一下,最近两个月,很多领导和同行都在反复「质问」我:曹大夫,很多医院医生仅仅观察了几例、十几例就能看出效果来了。你这里都200多例了,怎么还不知道效果呢?
我觉得这个问题很难回答,有些病人是有效的,但是如果我们继续观察,可能又是无效的。因此,要想回答每种疗法有效性的问题时,必须进行前瞻随机对照研究。
还有一个非常重要要求是「需要怀疑」,千万千万不能听说某种药有效,就敢给病人普遍临床应用。做为一个受过医学训练的人,贸然用药是很可怕的一件事情。事实上,我们经验的积累一定是建立在循证的基础上建起来的。因为我们专业主要是做肺炎方面,例如,一个医疗组一个月间收治了100个病人,这100个患者中,有几个真真正正能够把故事讲清楚的?病原学明确了吗?根据病原学药敏结果用药,患者是否像预期结果一样治疗好了?而且临床表现是不是和我们所掌握的基本规律是一致的?
在咱们临床看到的100个患者当中,我们真正能够拿到循证医学证据的,我不超过两位数,甚至个位数。哪怕一个月管的100个患者中,只有1个病人有循证医学证据,那就是非常宝贵的经验。这1个病人才叫经验,你管理其他99个病人都不会获得有价值的经验,而且有可能还把错误的经验当经验了,那就更加会伤害你的下一个病人。
通过这个采访 ,大家看明白了么?瑞德西韦在临床试验中是非常严谨的,严谨的到了严酷的地步,所以也难怪,疫情都快结束了,药物的效果到底行还是不行的结论都还只能靠猜,也反映了实际上在疫情爆发的时候做新药的试验实际上是风险极大的,有三个方面的困难,第一是竞争者众多,老药新用加上中草药,甚嚣尘上,不一而足,直接就把临床资源给抢了;第二,严格的试验设计,大家采访中也看到这个内容是非常严格的,也让试验的周期远远高于其他的试验,人家试验几十例就敢宣称有效,这边还要考虑病例的有效性,还要考虑循证医学的基础;第三就是患者入组的选择非常严格,主要是入组前不能使用任何药物的要求,而且长达8天不用任何药物的要求,实际上把大量的患者就直接pass掉了;加上双盲试验,很多患者认为对照组就是安慰剂,这种讲法,也让很多患者恐惧,害怕被分到对照组之后,成了死亡组,也极大地限制了患者的选择,在曹彬教授接受环球电视网的CGTN的采访中,他已经指出对照组接受的是标准治疗,而不是安慰剂治疗,可惜这消息出来的太晚,声音也太弱,远不及钟院士的声音,极大地影响了患者的入组,如果曹彬教授的临床试验组再不出声,这个瑞德西韦的临床试验是真的可能胎死腹中,流产结局的。
这个专访中,有些地方讲的也不是很清楚,但是也透露了几个关键的信息,第一就是瑞德西韦对于新冠病毒的有效性从病毒学角度是明显高于柯立芝等药物的;第二瑞德西韦的安全性是毋庸置疑的,它已经经过了临床1、2期的安全性试验,对人体是无害的,现在临床三期的重点是有效性测试;第三是瑞德西韦的临床试验实际上是可以看到后台数据的,也是可以随时做分析的,并不是只能等到4月27日才会有最终结果,双盲试验既然是人们设计的,就是可以时刻掌握进展,这里面既有避免悲剧性结果的考虑,也有及早发现药物有效,及时扩大使用的考虑。从这一点上来说,世界卫生组织专家组考察组离京时候新闻发布会上说只有瑞德西韦是有效的药物,就是有依据说的。
最后,看过了这么多消息,经过融汇理解,请您做一个小判断,您觉得瑞德西韦在临床上对于新冠病毒是否具有疗效?
- 03/07/2020 – be aware of fatality rate by age of CV.
Age of Coronavirus Deaths
COVID-19 Fatality Rate by AGE:
*Death Rate = (number of deaths / number of cases) = probability of dying if infected by the virus (%). This probability differs depending on the age group. The percentages shown below do not have to add up to 100%, as they do NOT represent share of deaths by age group. Rather, it represents, for a person in a given age group, the risk of dying if infected with COVID-19.
|
AGE
|
DEATH RATE confirmed cases |
DEATH RATE all cases |
|
80+ years old
|
21.9%
|
14.8%
|
|
70-79 years old
|
8.0%
|
|
|
60-69 years old
|
3.6%
|
|
|
50-59 years old
|
1.3%
|
|
|
40-49 years old
|
0.4%
|
|
|
30-39 years old
|
0.2%
|
|
|
20-29 years old
|
0.2%
|
|
|
10-19 years old
|
0.2%
|
|
| 0-9 years old |
no fatalities
|
- 02/25/2020 – A few virus related stocks are worth looking into
Investing in Biotech Stocks the Right Way During an Epidemic
A few factors need to be considered before betting on health care stocks
Many companies have already announced their plans to find a solution for the rapidly spreading COVID-19 virus. Notably, all these companies are billion-dollar pharmaceutical giants, which falls in line with the discoveries of this analysis. Below is a list of companies that have made some progress so far.
|
Company |
Comments |
|
GlaxoSmithKline (NYSE:GSK) |
On Feb. 24, the company announced a collaboration with a Chinese biotech firm, Clover Biopharmaceuticals, to develop a vaccine to fight the virus. |
|
Gilead Sciences (NASDAQ:GILD) |
The World Health Organization said on Monday that Gilead’s drug remdesivir is showing signs that it may help treat the virus. The company, in a statement made the same day, confirmed that clinical trials involving humans are currently underway and that results can be expected within a few weeks. |
|
Sanofi (SANOFI) |
On Feb. 18, the company announced a partnership with the U.S. government to find a solution to curb the spreading of the virus. |
|
Johnson & Johnson (NYSE:JNJ) |
The world’s largest drugmaker is working with the Biomedical Advanced Research and Development Authority to find a cure. |
|
Moderna (NASDAQ:MRNA) |
The company is developing a vaccine and the drug will be tested on humans within two and a half months, according to a statement released by the company on Feb. 24. |
Investors might be tempted to bet on these companies. However, there’s a better way to do the same thing as well.
There’s a simple way to gain exposure to a basket of biotech companies with attractive prospects. Investing in not one but many companies provides diversification benefits and can protect a porfolio from a severe downturn of a single stock. The iShares Nasdaq Biotechnology ETF (NASDAQ:IBB) primarily invests in large-cap health care stocks, which ensures that many of the companies that are actively seeking for a vaccine or a cure to fight COVID-19 are holdings of this exchange-traded fund.
- 02/18/2020 – some opportunities that I need to look into
5 Stocks That Could Rally if Coronavirus Slows
- 02/10/2020 – Trump thinks the virus will gone fair soon, we will see
Trump to Trish Regan: China has coronavirus under control
President doesn’t believe coronavirus will impact China buying of American goods
FOX Business’ Trish Regan spoke exclusively to President Trump minutes before his Keep America Great rally in Manchester, New Hampshire, on Monday about the growing concern over the coronavirus epidemic and the Fed’s rate decisions.
“I think China is very professionally run in the sense that they have everything under control,” Trump told Regan during “Trish Regan Primetime.” “I really believe they are going to have it under control fairly soon.”
Trump noted that warmer weather should help quell some of the germs, too, but in the meantime, the United States is sending World Health Organization experts to Wuhan, China, to observe and help as needed.
“I can tell you … we’re working with them,” Trump mentioned. “You know, we just sent some of our best people over there.”
- 02/02/2020 – great background article on coronavirus and model prediction
Here are the key factors on how bad it might get. (NYT) This is a very good background article covering contagion, how deadly, how long for symptoms, amount of travel by those infected, evaluation of a response, and the chances for a vaccine. The article has some great graphics showing the results of various models. Much depends upon how many people are infected by each new case.
- How contagious is the virus?
It seems moderately infectious, similar to SARS.
- How deadly is the virus?
It’s hard to know yet. But the fatality rate is probably less than 3 percent, much less than SARS.
- How long does it take to show symptoms?
Possibly between 2 to 14 days, allowing the illness to go undetected.
- How much have infected people traveled?
The virus spread quickly because it started in a transportation hub.
- How effective will the response be?
The W.H.O. has praised China’s efforts, but critics fear lockdown measures may not be enough.
- How long will it take to develop a vaccine?
A vaccine is still a year away — at minimum.
Barron’s reports on a model that zeroes in on China’s ability to quarantine its infected population.
If China can effectively quarantine its infected population by March, Cascend’s model forecasts, as few as 30,000 people will be infected, with 13,500 deaths. That is a lot of people, but as the Cascend report notes, the common flu kills 35,000 people a year and hospitalizes about 200,000 in the U.S. alone.
If the infected population isn’t quarantined by September, however, the number of infected people could reach 800,000, lifting the death toll to 200,000. The figures could reach 2.3 million infections and 600,000 deaths if the world can’t quarantine the infected population by the end of 2020.
- 02/02/2020 – due to the coronavirus fear, consumer sector is the one I should watch
Muddy Waters goes short Luckin Coffee
Coronavirus risk trade sweeps over the consumer sector
Jan. 31, 2020 10:16 AM ET|About: NIO Limited (NIO)|By: Clark Schultz, SA News Editor
It’s another down day for consumer companies with a presence in China as novel coronavirus developments continue to point to an extended period of reduced store traffic in the nation and outbound tourism to key markets
Nio (NIO -7.2%), Canada Goose (GOOS -4.9%), Luckin Coffee (LK -7%), Yum China (YUMC -2.4%), Ralph Lauren (RL -1.6%), PVH (PVH -2.5%), Tapestry (TPR -2.7%), Fossil (FOSL -6.4%) and Capri Holdings (CPRI -3%) are all lower on the day.
Some of the selling pressure is tied to concerns over Chinese spending even after the virus outbreak is contained.
- 02/02/2020 – should I invest in these biotech companies? such as GILD? or the companies to announce starting vaccine development?
news on GILD and coronavirus: Gilead Sciences Offers Experimental Drug for Coronavirus Treatments, Testing, The U.S. biotech firm has formalized agreement with China to conduct clinical trial of remdesivir
Investors rush into biotechs working on coronavirus vaccine
- “At least a dozen companies have informally or formally announced vaccine or drug development initiatives,” said Needham’s Alan Carr. “It appears at least a few programs will have moved into clinical testing within a few months.”
- Vir Biotechnology surged 110% in the past month, while Novavax gained 87% during the same period.
- Inovio Pharmaceuticals skyrocketed 34% in January after it said it’s in the process of developing a vaccine against the new deadly virus.
- Morgan Stanley is bullish on Moderna and Regeneron, which recently indicated plans to develop vaccine and produce therapeutic antibodies against the virus.
The deadly coronavirus worsened quickly this month, roiling the financial markets as investors fled risk assets amid concerns the outbreak would disrupt the global economy. Meanwhile, a number of biotech companies have ramped up vaccine or drug programs to battle the disease, causing investors to bid up their shares amid the market rout.
Traders are hoping their initiatives to develop treatment and prevention for the coronavirus could come to fruition at some point. Vir Biotechnology surged 111% in the past month, while Novavax gained 91% during the same period.
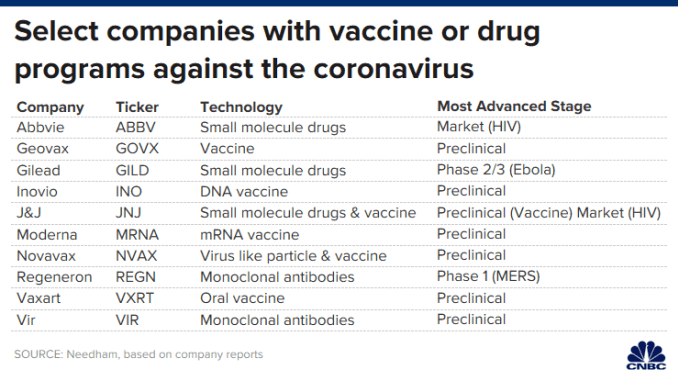
These programs include drugs already approved for other viral infections, unapproved drugs initially developed for other viruses, new monoclonal antibodies, new vaccines, the analyst pointed out.
Inovio Pharmaceuticals skyrocketed 37% in January as it said it’s in the process of developing a vaccine against the new deadly virus.
But analysts cautioned any commercial treatment from Inovio could be years away. Buyers in these stocks should beware as that could be the issue with any of these names amid the rampant speculation.
Morgan Stanley is bullish on Moderna, who recently indicated that it is working on a potential vaccine for the coronavirus.
“Moderna has a potential benefit over traditional vaccine makers in that once it has the sequences that code for the most immunogenic part of the virus’ surface proteins, or antigens, management can rapidly make a clinical development candidate,” the bank’s analyst Matthew Harrison said in a note.
The analyst also has high hopes for Regeneron, saying its screening technology could be used to potentially produce therapeutic antibodies against the virus.
“During the Ebola outbreak, Regeneron was able to move from development to validation of its therapeutic candidate in 6 months,” Harrison said.
- 01/27/2020 – If history is a lesson, the current fear might drop the SP500 from 5.8% to 12.9%.
five previous selloffs related to fears of spreading illness—SARS in 2003, avian flu in 2004, MERS in 2012, ebola in 2013 to 2014, and zika in 2015 to 2016—and would that the losses ranged from 5.8% for ebola (strange considering it’s been the deadliest of the diseases) to 12.9% for zika and 12.8% from SARS. Even if assuming the softest ebola-like landing, the S&P 500 is just 2.8% from its record high of 29348.10, hit on Jan. 17.
- 01/27/2020 – list of companies that will be significantly impacted by this fear:
- luxury retails: Estee Lauder, Nike, Tapestry, PVH and VF – but of course, consumers can always buy them online
- Travels: Marriott, Hilton and Hyatt
- restaurants: McDonald’s, Starbucks and Yum China
- Casino stocks: Wynn Resorts, Las Vegas Sands and MGM Resorts International
These US stocks have the most exposure to China’s consumer market getting hit by coronavirus fears
- Global brands Estee Lauder and Nike both generate 17% of their revenue from mainland China each year, Credit Suisse estimated based on the companies’ filings.
- Other apparel retailers with high China exposure include Tapestry, PVH and VF.
- Citi said American hotel companies that have “significant” exposure to China include Marriott, Hilton and Hyatt Hotels.
- McDonald’s, Starbucks and Yum China have all shut down some of their stores in China in response to the outbreak, leading analysts to worry about their near-term revenue picture.
“We estimate that Starbucks has the greatest exposure as measured by percentage of WW system revenues and operating income, followed by McDonald’s & Domino’s,” Guggenheim analyst Matthew DiFrisco said in a note.
The analyst said about 10% of Starbucks sales and up to 15% of its operating income come from China. There are about 3,300 McDonald’s locations in China, with 10% year-over-year unit growth, DiFrisco said
- 01/27/2020 – negative impact on travel stocks: InterContinental, Hawaiian Airlines, Royal Caribbean, British Airways -owner IAG and Lufthansa. In addition, I can look into travel ETFs.
Coronavirus Is No Small Threat to Travel Stocks
Past epidemics have had only a small impact on headline stock indexes, but some travel and tourism companies have been hit hard
term impact on the global economy or headline stock indexes.
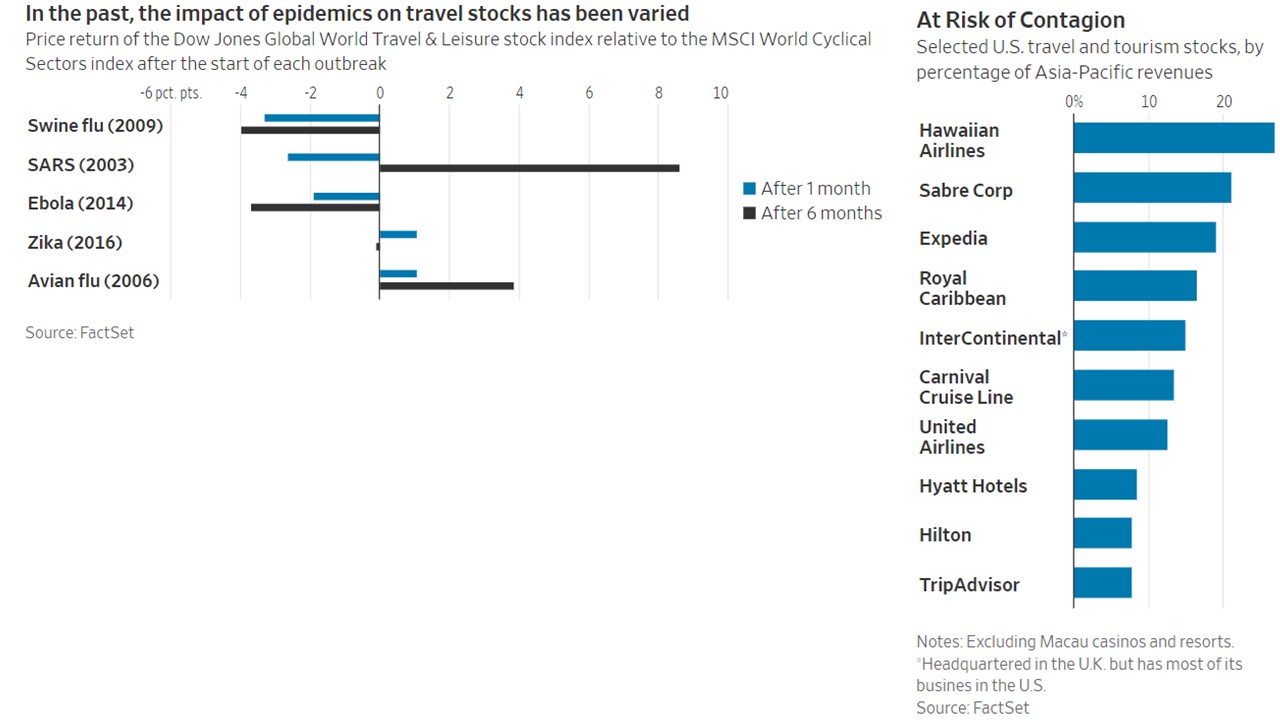
European airlines in particular are getting punished, given that they are a key gateway into and out of China. Shares in British Airways -owner IAG and Lufthansa are down 13% and 9%, respectively, since the start of last week.
In 2003, the operating profit of InterContinental, a hotel chain with a big Chinese business, dropped 55%, according to data provider FactSet—a result of both SARS and the Iraq war. China is generally a much bigger market for American companies than it was back then. U.S. travel companies that make a lot of revenue in the region now include Hawaiian Airlines and, to a lesser extent, United Airlines, as well as cruise lines like Royal Caribbean.
01/27/2020 – some basic knowledge about coronavirus