CV-19 fears – part II
- 12/20/2021 – New York City expects omicron infections to rapidly surge but peak in a matter of weeks, mayor says
- “We’re going to see a really fast upsurge in cases, we’re going to see a lot of New Yorkers affected by omicron,” New York City Mayor Bill de Blasio said.
- However, the city expects the omicron surge to peak in a matter of weeks, de Blasio said.
- The mayor said most New Yorkers who have caught omicron so far are showing mild symptoms.
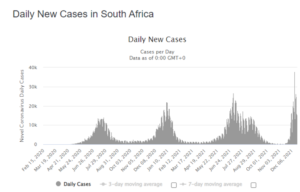
- 12/17/2021 – encouraging news from South Africa. Will we have a few weeks’ surge on CV?
They’re Already Past the Peak in South Africa’s Omicron Ground Zero
Even as the world panics over the rapid spread of the Omicron variant of COVID-19, the health ministry in South Africa has delivered some encouraging news. On Friday, South Africa’s Minister of Health Joe Phaahla said that hospitalizations are down with Omicron cases compared to previous waves like the deadly Delta surge, and that people in the hospital rarely need oxygen and are less likely to die from the disease.
- 12/15/2021 – Fauci says Covid boosters work against omicron, no need for variant-specific third shot
- White House chief medical advisor Dr. Anthony Fauci said the primary two-dose vaccination series from Pfizer and BioNTech is significantly compromised by omicron.
- However, two shots still offer considerable protection against severe disease, he said.
- A booster dose increases protection against symptomatic disease to 75%, Fauci said.
- 12/03/2021 – wait and see the effect of Omicron
Omicron will likely ‘dominate and overwhelm’ the world in 3-6 months, doctor says
- “Frankly, omicron will dominate and overwhelm the whole world in three to six months,” Singapore doctor Leong Hoe Nam told CNBC’s “Street Signs Asia.”
- New vaccines targeting omicron are a “nice idea” but won’t be practical because of the transmissibility of the strain, he said.
- Experts don’t know exactly how contagious the highly mutated omicron variant is, but the virus’ spike protein — which binds to human cells — has mutations associated with higher transmission and a decrease in antibody protection.
- 11/29/2021 – Gottlieb is confident on vaccine efficacy against Omicron
Dr. Scott Gottlieb: The vaccines might be sufficient to control spread of new Covid variant
Gottlieb says Omicron variant “almost definitely” already in U.S.
Dr. Scott Gottlieb: Omicron Covid variant might have circulated for ‘quite some time’
- 11/29/2021 – US government is in action to deal with Omicron
Biden to direct regulators to use ‘fastest process available” to clear vaccines against Omicron
- President Joe Biden says that his administration is working with vaccine makers, Pfizer (NYSE:PFE), Moderna (NASDAQ:MRNA), and Johnson & Johnson (NYSE:JNJ), to devise contingency plans if new vaccines or boosters are needed for the new COVID-19 variant, which was first detected in South Africa.
- Speaking on Monday at the White House, President noted that the current generation of vaccines offers at least some degree of protection against the Omicron variant, classified by the World Health Organization (WHO) as a global “variant of concern” last week.
- However, booster shots “strengthen that protection significantly,” CNBC quoted President Biden as saying.
- He also promised Americans that the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) will be guided to use the “fastest process available without cutting any corners” for regulatory clearance of the necessary vaccines.
- 11/29/2021 – Pfizer CEO is very confident on CV pill and vaccine
Pfizer CEO has ‘high level of confidence’ COVID-19 pill will be effective against omicron (msn.com)
Pfizer CEO confident Covid treatment pill effective against omicron variant (cnbc.com)
- Pfizer CEO Albert Bourla said he has a “very high level of confidence” that the company’s Covid treatment pill is effective against the omicron variant.
- Bourla said Pfizer has already started work on a new vaccine and it could be ready in less than 100 days.
- 11/29/2021 – seeking alpha’s comments on Omicron – not serious concern. we will have better answer in two weeks.
Wall Street Breakfast: The Omicron Edition
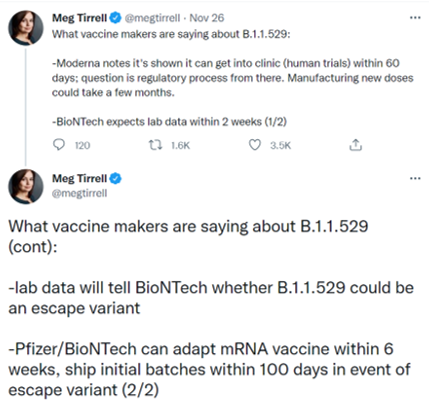
Vaccine makers have thus far been hesitant to tweak existing coronavirus jabs due to fears it could become a game of whack-a-mole, but a super strain may change that sentiment. Testing involving Omicron is already underway among the big manufacturers, with several saying it would take about two weeks to establish whether the new variant renders their shots less effective. On Friday, South Africa’s health minister said he expects current jabs to still offer protection against severe illness and death from Omicron, though they might be less effective at preventing infections and milder disease.
In case it doesn’t: Pfizer (NYSE:PFE) and partner BioNTech (NASDAQ:BNTX) said they would be able to adapt their vaccines within six weeks and ship initial batches within 100 days. Moderna can meanwhile get a new construct into human trials in less than 60 days, though manufacturing would take a few months. Any new candidates would also need to clear the regulatory process, getting a green light from the Food and Drug Administration.
In terms of therapeutics, Pfizer’s (PFE) Paxlovid and Merck’s (NYSE:MRK) molnupiravir are expected to target parts of the virus that are not changed in Omicron, and could be all the more important if vaccine-induced and natural immunity are threatened. Both drugmakers have licensed their antiviral pills to other manufacturers, so they should be widely available if needed. The Biden administration has even ordered 13M courses of the two drugs after seeing significant success in trials at preventing severe illness in high-risk groups.
Outlook: Regarding manufactured antibodies, Omicron’s mutations are likely to render some treatments ineffective, according to Dr. David Ho, professor of microbiology and immunology at Columbia University. Investors are particularly worried about antibody drugs from Regeneron (NASDAQ:REGN) and Eli Lilly (NYSE:LLY), but some think Vir Biotechnology (NASDAQ:VIR) and AstraZeneca’s (NASDAQ:AZN) could hold up better against this variant due to the way the antibodies are constructed to target the spike proteins. The new variant might also dull the impact of monoclonal antibody treatment because of those same mutations, declared Dr. Wendy Barclay, head of the G2P-UK National Virology Consortium.
Doctor who helped detect new COVID-19 variant calls its symptoms unusual but mild – The Telegraph
- The first South African physician to raise the alarm over the new COVID-19 variant in South Africa called its symptoms unusual but mild, according to The Telegraph.
- The newly found variant, named Omicron and classified as a “variant of concern” by the World Health Organization (WHO), sparked a selloff in financial markets on Friday and triggered stricter travel restrictions across the globe.
- Early this month, South African doctor Dr. Angelique Coetzee was concerned over a potential new variant after COVID-19 patients turned up at her private practice in Pretoria with symptoms that did not make sense initially.
- “Their symptoms were so different and so mild from those I had treated before,” remarked Dr. Coetzee, who is also the chairwoman of the South African Medical Association. None of the patients had experienced a loss of taste or smell, considered as hallmarks of the disease.
- While there were about two dozen COVID-19 positive cases with symptoms of the new variant, many of the patients were healthy young men who showed up “feeling so tired.” About half of them were unvaccinated, Dr. Coetzee added.
How Omicron Variant Rattled the World in One Week – WSJ
Speedy discovery, announcement and global response shows new phase in fight against Covid-19, as health officials hunt for variants that could evade vaccines
- 11/29/2021 – Tilson thinks Omicron is not a serious concern. if we need another vaccine, we can do this incredibly quickly. Thanks to the new biotechnology, mRNA vaccines are really easy to alter. Once the minor change is made, only 2 dozen people need to enroll in a trial to make sure the updated vaccine works. Then it can be distributed to arms. Because the change is small, an updated vaccine doesn’t need Phase III trials and/or regularity approval. So, this whole process should take a max of 6 weeks. We haven’t heard from Moderna or Pfizer if they’ve started creating an updated vaccine, but I guarantee conversations have started behind closed doors.
Coronavirus Update 11/27/21 – great comments from Tilson
1) In my October 11 e-mail, I wrote:
If you were to tell me that the stock market will crash by 25% or more over the next year and asked me to identify the possible reasons for it, here are my top three:
a) Rising inflation and/or debt levels worldwide will roil debt markets and spook equity investors.
b) A new COVID-19 variant will emerge that is resistant to the current vaccines, leading to a surge in deaths and a return to lockdowns.
c) We will get into a shooting war with China – likely over Taiwan.
Sure enough, the second one – the rise of the omicron variant of the coronavirus – is spooking investors, leading to the worst Black Friday for the markets in 90 years.
It certainly has me reevaluating what I wrote in Wednesday’s e-mail, where I laid out the case for why I “highly doubt[ed]” that “a resurgence of the pandemic [would] lead to a reimposition of lockdowns.”
I’ve been doing a lot of reading about the new variant over the weekend and shared what I found with my coronavirus e-mail list on Saturday (to join it, simply send a blank e-mail to: cv-subscribe@mailer.kasecapital.com). You can read my full e-mail here. Here’s a summary:
The world is freaking out about the new omicron variant. Is this warranted? It’s hard to say because we don’t know anything for sure right now, so I suppose it’s better to be safe than sorry until we know more.
Before I dive into a discussion of the new variant, I want to underscore that it’s now even more of a slam dunk case to get the booster as soon as you can…
In summary, I think this article is exactly right: don’t think of this as getting a two-shot vaccination and then an optional booster: We Screwed Up, This Is Really a Three-Dose Vaccine.
Here’s an op-ed in the Washington Post making the same point: What the CDC got wrong with COVID-19 booster shots…
Here’s Dr. Kevin Maki’s summary of the situation today:
Regarding the new omicron variant, I think it is too early to draw any firm conclusions. The types and locations of the mutations raise concerns about transmissibility and [the] ability to evade vaccine-induced immunity. We have very little epidemiology to suggest that this variant is truly more transmissible than the delta variant. If it is, that would be of concern because a more transmissible variant would likely become dominant, as happened with the U.K. variant then the delta variant.
Even if omicron has some ability to evade vaccine-induced immunity (B cell and T cell), that does not mean that the vaccines will be impotent against this variant, only that there may be less protection. Even if protection with the new variant starts out lower (maybe 50%-60%), it may still do a decent job of protecting against severe outcomes.
We should know in a couple of weeks how well serum from vaccinated individuals neutralizes this variant. Also, epidemiologic data will help to establish the severity of illness for omicron breakthrough infections now that we will have variant-specific surveillance. It would take at least four months to produce a booster specific for this variant.
Finally, in a few weeks, the FDA (Food and Drug Administration) should grant emergency use authorization for oral antivirals. The Pfizer (PFE) cocktail appears to be particularly good.
The combination of readily available testing, vaccines, and therapeutics (oral antivirals and monoclonal antibodies) mean that we are in a much better situation than we were at the start of the pandemic. As a result, I am not overly concerned right now, although that could change if new data emerges showing that the omicron variant has a higher ability to evade vaccine-induced immunity.
Lastly, here’s the latest from front-page stories in today’s New York Times and Wall Street Journal:
- Will the Vaccines Stop Omicron? Scientists Are Racing to Find Out.
- How Omicron Variant Rattled the World in One Week
here’s another good summary by Katelyn Jetelina, a blogger and Assistant Professor at The University of Texas Health Science Center at Houston: New Concerning Variant: B.1.1.529. She first addresses the reasons everyone is so concerned, and then concludes with some reasons for optimism:
There is some good news though
First, we can detect B.1.1.529 on a PCR test. This typically isn’t the case. Usually a swab would have to go to a special lab for genome sequencing to know which variant caused the infection. However, it looks like B.1.1.529 has a special signal like Alpha on the PCR directly. For example, when the PCR is positive it lights up two channels instead of three channels, indicating that it’s B.1.1.529. This is amazing news because it means we can track this virus much easier and much quicker around the world.
Second, we caught this virus incredibly early. I can’t stress enough how amazing South Africa has been on communicating and taking hold of the situation. Because of their swift response, scientists around the world are already working together to decode this new threat. Early detection means that we have a surveillance system in place and it’s working.
Third, if we need another vaccine, we can do this incredibly quickly. Thanks to the new biotechnology, mRNA vaccines are really easy to alter. Once the minor change is made, only 2 dozen people need to enroll in a trial to make sure the updated vaccine works. Then it can be distributed to arms. Because the change is small, an updated vaccine doesn’t need Phase III trials and/or regularity approval. So, this whole process should take a max of 6 weeks. We haven’t heard from Moderna or Pfizer if they’ve started creating an updated vaccine, but I guarantee conversations have started behind closed doors.
Bottom Line: There’s still so much that we don’t know but what we do know is incredibly concerning. We are in a lull right now as we wait for scientific evidence to answer two questions as soon as possible:
Does B.1.1.529 escape vaccines like we fear?
Does B.1.1.529 continue to outcompete Delta like we’re seeing in South Africa?
Once we have answers to these two questions, we’ll know the next step. Stay tuned.
- 11/27/2021 – Pfizer, Moderna, J&J, Novavax are moving fast to counter the surge of Omicron variant
Pfizer, Moderna say they can quickly adapt COVID-19 vaccines for Omicron variant
- Pfizer (PFE +5.7%) and Moderna (MRNA +20.2%) say that they can make changes to their current mRNA vaccines to ensure they are effective against the Nu variant, CNBC reports.
- Pfizer/BioNTech (BNTX +14.7%) say that they can adapt their vaccines within 60 days and ship the first batches in 100 days.
- Moderna says that it can begin human testing on an adapted vaccine in 60 days, but noted that the timeline after that is subject to regulatory processes. They added manufacturing doses could take several months.
- Johnson & Johnson (JNJ -0.2%) has begun testing its vaccine’s effectivness against the Nu variant, CBS News reports.
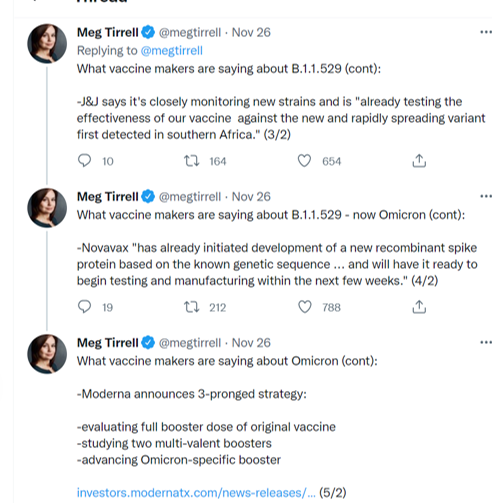
Moderna Announces Strategy to Address Omicron (B.1.1.529) SARS-CoV-2 Variant
Moderna Announces Strategy to Address Omicron (B.1.1.529) SARS-CoV-2 Variant (pdf file)
Third, Moderna will rapidly advance an Omicron-specific booster candidate (mRNA-1273.529). This candidate is part of the Company’s strategy to advance variant-specific candidates for a subset of variants of significant concern. During 2021 this has already included Beta- and Delta-specific boosters. The Company has repeatedly demonstrated the ability to advance new candidates to clinical testing in 60-90 days.
“From the beginning, we have said that as we seek to defeat the pandemic, it is imperative that we are proactive as the virus evolves. The mutations in the Omicron variant are concerning and for several days, we have been moving as fast as possible to execute our strategy to address this variant,” said Stéphane Bancel, Chief Executive Officer of Moderna. “We have three lines of defense that we are advancing in parallel: we have already evaluated a higher dose booster of mRNA-1273 (100 µg), second, we are already studying two multi-valent booster candidates in the clinic that were designed to anticipate mutations such as those that have emerged in the Omicron variant and data is expected in the coming weeks, and third, we are rapidly advancing a Omicron-specific booster candidate (mRNA-1273.529).”
- 11/25/2021 – watch out the new CV variant. The U.K. announced it would ban flights from six African countries, including South Africa, starting midday Friday.
- WHO is monitoring a new Covid variant, scheduling a special meeting Friday to discuss what it may mean for vaccines and treatments, officials said.
- The variant, called B.1.1.529, has been detected in South Africa in small numbers.
- South African scientists have detected 30 mutations to the spike protein, the part of the virus that binds to cells in the body, which could have implications for vaccine efficacy and transmissibility.
The U.K. announced it would ban flights from six African countries, including South Africa, starting midday Friday.
The UK Health Security Agency “is investigating a new variant,” Health Secretary Sajid Javid said Thursday in a tweet announcing the travel restrictions. “More data is needed but we’re taking precautions now.”
- 11/19/2021 – will US have another CV-19 wave? If so, this will significantly affect bond yield, oil price, small business, financial, retails
Covid cases rise yet again in U.S. ahead of Thanksgiving holiday
Doctors are urging caution to prevent Covid-19 outbreaks as cases rise nationwide following a nearly three-week plateau and Americans prepare to celebrate Thanksgiving with friends and family next week.
The U.S. reported a seven-day average of nearly 95,000 new Covid infections Thursday, up 31% over the past two weeks, according to a CNBC analysis of data from Johns Hopkins University. Cases across the country declined for weeks this fall before hovering between 70,000 and 75,000 per day beginning in late October, down more than 50% from the peak of the delta surge that ravaged the U.S. this summer.
The combination of Thanksgiving, Christmas and falling temperatures makes this time of year “the perfect storm” for Covid, Dr. Bruce Farber, chief of infectious disease at Northwell Health in New York, told CNBC.
The Northeast’s highly dense cities and the Midwest’s colder temperatures – compared with the South, where cases have plunged as more comfortable weather rolls in – could help explain those regional differences, Panettieri noted.
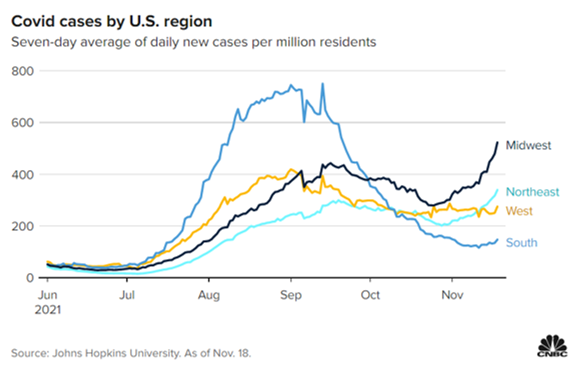
Dr. Fauci says Covid cases are starting to climb in some areas of the U.S
- Fauci’s comments came just a day after the country reported a seven-day average of more than 82,000 new cases, up 11% from the week before.
- Nationwide cases were down 57% last week from the delta wave’s peak this summer, but an influx of Covid patients in the Midwest and Northeast are fueling the sudden increase.
- Cases and hospitalizations have fallen sharply in the South, where the delta wave hit earliest and hardest over the summer.
- 11/19/2021 – CV-19 re-surge in Europe, be aware. Experts expect more nations to face the same dilemma as temperatures continue to fall across Europe, pushing people indoors where the Delta variant of the virus has proven to be highly contagious, including for people who got their vaccines months ago and whose immunity has waned.
Covid-19 Surge Prompts Renewed Lockdown in Parts of Europe – WSJ
In U-turn, officials in Germany and elsewhere impose new measures following rise in infections among vaccinated people
BERLIN—Parts of Europe are again locking down to prevent hospitals from becoming overwhelmed by a steep rise in Covid-19 patients as data shows a rise in infections among vaccinated people who are passing on the disease.
The German state of Saxony declared it would go into a partial lockdown for two or three weeks starting Monday, closing bars, restaurants and clubs and canceling all large events. Other badly hit German states are considering similar measures and may announce them in the coming days.
Testing for vaccinated and unvaccinated alike would become mandatory in some regions once hospitalizations reach a given threshold, German authorities said on Thursday. Vaccinations will now be made mandatory for healthcare workers.
Europe is currently divided into roughly four zones. Southern and Western Europe, including Portugal, Spain, Italy and to a lesser extent France, have seen infections rise from a very low base. Northern and Central European countries from the Netherlands to Belgium, Germany and Austria, which have comparable vaccination rates, have seen new cases reach record highs in recent weeks and have a crunch on hospitals.
Parts of Eastern Europe, including Bulgaria and Romania, where vaccination rates are comparatively low, are facing a devastating winter, with record deaths and hospitalizations. Romanian Covid-19 patients are being airlifted into other EU countries after the healthcare system reached its capacity.
The U.K. is in a category of its own. While case numbers, hospitalizations and deaths roughly match the European Union average, the government there has pledged not to reintroduce restrictions. The country had some of the worst death tolls in Europe. Around 14,000 people have died with a Covid-19 infection there since Britain, with a population of 67 million, abandoned all measures on July 15. Germany, with over 83 million people, recorded just under 7,000 coronavirus-related deaths in the same period.
Austria Becomes First in Europe to Impose Vaccine Mandate, Return to Lockdown – WSJ
In a warning sign for other Western countries, the resurgence of the virus comes even though two thirds of the population have been vaccinated
Austria became the first European country to introduce a general vaccination mandate and return to a nationwide lockdown in reaction to a rapid rise in Covid-19 infections and hospitalizations despite mass immunization.
The decision comes as a warning sign to other Western countries, including the U.S., that had hoped to put the pandemic behind them thanks to successful vaccination campaigns.
- 11/05/2021 – 89% efficacy Covid antiviral pill will be available by January, this along with Merck and Ridgeback Biotherapeutics’s pill can become a game changer.
Pfizer board member Gottlieb says the Covid pandemic could be over in the U.S. by January
Gottlieb’s comments came in the wake of data from Pfizer that indicated its Covid antiviral pill, when paired with an HIV medication, slashed the potential for hospitalization or death by 89% in adults at risk for severe complications. Combining the pill with an HIV medication slowed the metabolism, allowing the Covid antiviral to work longer in the body.
Pfizer CEO Albert Bourla said in an interview Friday morning with “Squawk Box” before Gottlieb spoke that the company will submit data on the therapeutic to the FDA before Thanksgiving.
Gottlieb said Pfizer’s new drug shouldn’t be considered a substitute for vaccinating more Americans against the virus, adding that he thought periodic Covid vaccinations and changes to the vaccines could be necessary moving forward. But he noted that antivirals could help treat cases in a range of high-risk individuals.
“When you have therapeutics that are this effective, that can be a backstop for people for whom vaccines don’t work, people who have breakthrough infections – there’s pills being studied in that setting,” Gottlieb said. “It really is a backstop against death and disease from this infection.”
Pfizer’s announcement arrives a month after Merck and Ridgeback Biotherapeutics said they created an antiviral that cut Covid hospitalizations and deaths by 50% in patients with up to moderate cases. The United Kingdom cleared Merck’s treatment Thursday.
Pfizer says its Covid pill with HIV drug cuts the risk of hospitalization or death by 89%
- Pfizer said its Covid-19 pill, used with an HIV drug, cut the risk of hospitalization or death by 89% in high-risk adults who’ve been exposed to the virus.
- It’s now the second antiviral pill behind Merck’s to demonstrate strong effectiveness for treating Covid at the first sign of illness.
- Pfizer said it plans to submit its data to the Food and Drug Administration “as soon as possible.”
- 11/04/2021 – watch out the CV situation in Europe.The two main reasons the WHO’s Kluge gave for Europe’s soaring case numbers were insufficient vaccination coverage and the relaxation of public health and social measures.There have been concerns about the increasing prevalence of a new mutation of the highly infectious delta variant as well as sluggish vaccination campaigns, booster vaccination drives and the onset of the winter season where viruses spread more easily with more people convening indoors.
WHO warns that Europe is once again at the epicenter of the Covid pandemic
- Europe is facing a worrying resurgence in Covid-19 cases, according to the the World Health Organization.
- The two main reasons the WHO gave for Europe’s soaring case numbers were insufficient vaccination coverage and the relaxation of public health and social measures.
- Leading German health officials warned on Wednesday that the country was entering a fourth wave of the pandemic.
- 11/01/2021 – Kids Covid vaccination program will be ‘fully up and running’ next week, White House says
- “Starting the week of Nov. 8, the kids vaccination program will be fully up and running,” White House coronavirus response coordinator Jeff Zients said.
- The Biden administration has already begun transferring 15 million vaccine doses from Pfizer to facilitate immunizations at pediatricians’ offices, pharmacies, hospitals and health centers.
- Vaccinations for 5- to 11-year-olds could begin this week, Zients said.
- 10/11/2021 – this is positive for energy and other industries (Las vagas) which are still not come back yet
Dr. Scott Gottlieb says Merck’s Covid pill ‘can make a real difference’
- Merck said it asked the FDA to authorize emergency use of its experimental antiviral pill to treat mild to moderate Covid-19 in adults.
- “The topline data from this Merck study was probably the best treatment effect we’ve seen from orally available antiviral drug in the treatment of any respiratory pathogen, so this can make a real difference,” said Dr. Scott Gottlieb.
- 09/19/2021 – The antiviral pills could come by the end of the year
Pills to Battle Covid Are Coming. These Companies Stand to Gain
the next generation of Covid-19 antivirals is on the way, and a pill to treat—or even prevent—Covid-19 could be available by the end of the year. Merck (MRK), Pfizer (PFE), and the biotech Atea Pharmaceuticals (AVIR) each expect late-stage data on an oral Covid-19 antiviral in the coming months. If the data are positive, the drugs provide a major opportunity for the companies—one that investors should not ignore.
The antivirals may not be effective enough to stop a Covid-19 infection in its tracks.
Still, if they show even moderate efficacy, they will play a major role as the global fight against the virus shifts to a long-term grind against an endemic threat. A prescription Covid-19 antiviral that could be taken at home as a pill would be in great demand around the world.
Analysts at Jefferies have said that an effective, convenient treatment for Covid-19 could be a $10 billion-a-year drug. That treatment will compete with monoclonal antibody therapies for Covid-19 from Regeneron Pharmaceuticals (REGN), GlaxoSmithKline (GSK), and others, which work well but, like Veklury, are generally administered as an intravenous infusion, making them challenging for widespread use.
In a recent note, Jefferies analyst Michael Yee wrote that he expected antivirals to be only incrementally effective. Still, he says there will be a need for them “for the foreseeable future.”
Given the potential size of the market, positive data or an emergency-use authorization for any of the three oral antivirals could give the shares of its maker a tremendous boost.
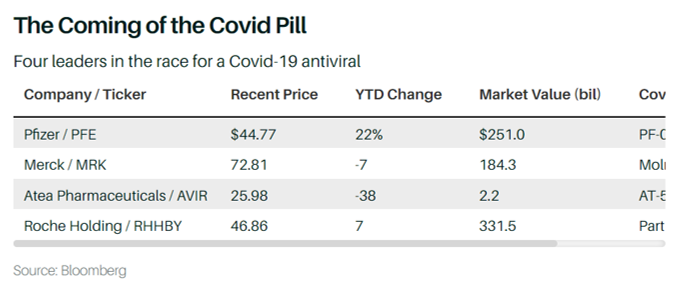
For Atea, a biotechnology company developing a range of oral antivirals, the effect on the share price could be the most significant of them all. Its Covid-19 antiviral, AT-527, is being studied in a number of trials, including a Phase 3 trial in nonhospitalized patients expected to produce data later this year. The company has teamed up with Roche Holding (RHHBY), which will have the rights to sell the drug outside of the U.S. SVB Leerink analyst Roanna Ruiz recently set a $60 price target on Atea shares, more than double their recent price of around $26.
- 09/14/2021 – vaccine for kids are coming
Pfizer CEO says Covid vaccine data for kids under age 5 may come in late October
- Pfizer expects to release clinical trial data on how well its Covid-19 vaccine works in 6-month to 5-year old children as early as the end of October, CEO Albert Bourla said Tuesday.
- Covid vaccine data for kids between ages 5 to 11 will come much sooner, he said, potentially ready to be submitted to the FDA by the end of this month.
- “Then, it is up to the FDA to take their time, and then make a decision,” Bourla said.
On Friday, FDA officials said they were “working around the clock” to support the approvals of Covid vaccines for kids under age 12.
Dr. Peter Marks, the agency’s top vaccine regulator, said last month the agency would move as “swiftly” as possible on approving the shot for kids under 12 once the companies submit data.
“Currently, there are still trials ongoing and so the agency has to wait for the company to submit the data for those trials,” he said on Aug. 23. “We certainly want to make sure that we get it right.”
- 09/13/2021 – The US is nearing immunity from COVID-19
Despite media claims that “We Can’t Turn the Corner on COVID,” the numbers of COVID-19 cases, new hospitalizations, and deaths nationwide peaked and started to decline around the beginning of September. The combination of this milestone, new findings from the Centers for Disease Control and Prevention showing widespread levels of vaccination and natural immunity, and improved availability of treatments suggests that, outside of isolated pockets, COVID-19 is likely to become a diminishing health risk in the United States.
- 09/07/2021 – watch out Mu in US
- A new COVID-19 variant called Mu might be able to evade the immunity people get from vaccines, Insider reported.
- The Mu variant has been detected in 47 US states and the District of Columbia, according to data from Outbreak.info.
- Only Nebraska, Vermont, and South Dakota are yet to detect a case, the data says.
- 09/07/2021 – The Delta variant may have been slow to gain traction in South America because there is already so much natural immunity in the region from people having contracted the virus.
South America, Where Lambda and Mu Variants Broke Out, Sees COVID Cases Plummet
Almost every country in South America has seen a drop in new COVID-19 case numbers—with the situation marking a stark contrast to tallies seen just weeks ago.
The continent is where some of the COVID-19 variants of interest (VOIs) were first found, including the Lambda and Mu variants—which were first detected in Peru in December 2020 and Colombia in January 2021 respectively.
Commenting on the this drop in cases across South America, Carla Domingues, an epidemiologist who ran Brazil’s immunization program until 2019, told The New York Times: “Now the situation has cooled across South America. It’s a phenomenon we don’t know how to explain.”
Jairo Méndez Rico, a viral diseases expert advising the World Health Organization, told the paper that the Delta variant may have been slow to gain traction in South America because there is already so much natural immunity in the region from people having contracted the virus.
- 09/06/2021 -Pfizer vaccine for trial results for 5- to 12-year-olds could come by the end of this month, which could mean shots aren’t authorized for use until October or November. Data for younger children could come in October. Moderna Inc. expects to seek emergency use for 6- to 12-year-olds by the end of this year, and early next year for children 6 months to less than 6 years, a company spokeswoman said.
Why a Covid-19 Vaccine for Children Is Taking So Long – WSJ
Researchers testing shots in children face serious challenges, starting with making the lower dose under study
The challenges are contributing to the long timelines testing the shots in children. Pfizer-BioNTech trial results for 5- to 12-year-olds could come by the end of this month, which could mean shots aren’t authorized for use until October or November, months after they were cleared for adolescents. Data for younger children could come in October.
Moderna Inc. expects to seek emergency use for 6- to 12-year-olds by the end of this year, and early next year for children 6 months to less than 6 years, a company spokeswoman said.
- 09/02/2021 – Fauci’s comments on MU. Lab in-vitro data shows that MU has the ability to evade antibodies, but there isn’t a lot of clinical data to suggest that. I need to pay close attention to this.
Fauci says the new mu Covid strain isn’t an immediate threat in the U.S.
- The new Covid-19 variant “mu” is not an immediate threat to the United States, federal health officials said Thursday.
- Mu — also known by scientists as B.1.621 — was added to the WHO’s list of variants “of interest” on Monday.
- “We’re keeping a very close eye on it,” Fauci said.
Mu — also known by scientists as B.1.621 — was added to the WHO’s list of variants “of interest” on Monday, the international health organization said in its weekly Covid epidemiological report published late Tuesday.
“This variant has a constellation of mutations that suggests that it would evade certain antibodies, not only monoclonal antibodies, but vaccine and convalescent serum induced antibodies,” Fauci said. “But there isn’t a lot of clinical data to suggest that, it is mostly laboratory in-vitro data.”
Even though Covid-19 vaccines were created with the original Covid strain in mind, the vaccines are still very effective against the delta strain.
“Remember, even when you have variants that do diminish somewhat the efficacy of vaccines, the vaccines still are quite effective against variants of that time,” Fauci said.
- 09/01/2021 – Vaccines are still very effective, but I still need to watch out the new variant and need to response fast
Covid vaccines remain ‘stunningly effective,’ even as delta concerns grow
- Covid-19 vaccines are still “stunningly effective” despite fears that immunity may dwindle over time, experts have said.
- There have been some concerns about the efficacy of Covid-19 vaccines after a number of recent studies indicated a growing number of “breakthrough” Covid cases among the fully vaccinated.
- Studies have shown that the fully vaccinated are still highly protected against severe infection, hospitalization and death caused by the virus.
WHO presses world leaders to hold off on Covid vaccine booster shots through September
WHO says it is monitoring a new Covid variant called ‘mu’
- The World Health Organization is monitoring a new coronavirus variant called “mu.”
- It has mutations that have the potential to evade immunity provided by a previous Covid-19 infection or vaccination, the WHO said.
- The new variant was first identified in Colombia but has since been confirmed in at least 39 countries, according to the agency.
- 08/30/2021 – Pfizer (NYSE:PFE) and BioNTech (NASDAQ:BNTX) COVID-19 vaccine could be granted the FDA’s emergency use authorization for children aged 5 to 11 years in late fall or early winter, according to Dr. Scott Gottlieb. In June, Pfizer (PFE) initiated a Phase 2/3 study to evaluate the vaccine in children aged six months to 11 years. The full dataset from the study was expected by the end of the year
Pfizer/BioNTech COVID-19 shot could win regulatory nod for kids by winter – Dr. Scott Gottlieb
- Pfizer (NYSE:PFE) and BioNTech (NASDAQ:BNTX) COVID-19 vaccine could be granted the FDA’s emergency use authorization for children aged 5 to 11 years in late fall or early winter, according to Dr. Scott Gottlieb.
- Gottlieb, a board member of Pfizer (PFE), thinks that the data from the clinical study for the vaccine targeting the age category “should be available in September,” followed by an FDA submission that month.
- “The application probably isn’t going to be submitted until some point in October,” Dr. Gottlieb, who led the FDA from 2017 to 2019, said on CNBC.
- “If the FDA sticks to its normal timeline, in terms of how it reviews these applications, you would expect that review to be a four-to-six week review for a potentially emergency use authorization, so that puts you on a timeline where you’re late fall, early winter,” he added.
- The messenger-RNA-based COVID-19 vaccine developed by Pfizer (PFE) in partnership with BioNTech (BNTX) recently won full FDA approval for those aged 16 and above. It is currently available in the U.S. under EUA for adolescents aged 12 – 15 years.
- In June, Pfizer (PFE) initiated a Phase 2/3 study to evaluate the vaccine in children aged six months to 11 years. The full dataset from the study was expected by the end of the year, the company said at the time.
- 08/28/2021 – Good to reach hospitalization peak?
U.S. Covid-19 Hospitalizations Approach a Peak as Delta Variant Spreads – WSJ
- 08/24/2021 – Pfizer CEO said they have not found any CV variant can resist the vaccine yet, but it was likely a vaccine-resistant variant would emerge. And Pfizer has the ability to create new vaccine in 95 days. I need to watch out this development.
Pfizer CEO says a vaccine-resistant coronavirus variant is ‘likely’ to emerge
- Pfizer CEO Albert Bourla told Fox it was likely a vaccine-resistant variant would emerge.
- Bourla said Pfizer could make a shot tailor-made for such a variant within 95 days of its discovery.
- The CDC director said the virus could be “a few mutations” away from evolving to evade vaccines.
- 08/23/2021 – the full approval for 12-to-15-year-olds and 12-year-old under will come before the under of this year according to Dr. Fauci
Dr. Fauci on FDA approval for Pfizer, Moderna and J&J shots
Judy Woodruff:
As we know, Dr. Fauci, this applies only to people who are 16 and up.
So I want to ask you about those 12-to-15-year-olds who would still be taking the vaccine under emergency authorization. We heard today from Dr. Peter Hotez. I’m sure you know him. He is a dean, professor of pediatrics and molecular virology, microbiology at the Baylor College of Medicine, works in vaccine development.
He said mandates — he said, by excluding this group of young people 12 to 15, mandates for middle schools may be out the window. And he spoke about how many pediatricians are still having a hard time convincing families that it’s safe.
Dr. Anthony Fauci:
Well, I mean, mandates will be very difficult, I believe, in some situations when you don’t have the full approval.
But they looked at the data that was presented by the company. And the data were for 16 and older. The 12 to 15 can still get the vaccine through EUA, which is the way that were getting it all along. But that is the point.
Sometimes, entities, organizations, schools are hesitant to mandate because it doesn’t have the full approval. But I believe, Judy, that you may get an indirect effect. I understand what Peter’s saying. He’s a good friend and a really highly competent person. I understand what he’s saying.
But my feeling is, the very fact that you have a full approval of a BLA for 16 and older, that would even indirectly give some backing for mandates for anybody who is approved either through an EUA or through the BLA.
Judy Woodruff:
What is your best understanding, Dr. Fauci, of when we are going to see full approval for the 12-to-15-year-olds and any approval for children who are younger than 12?
Dr. Anthony Fauci:
Well, let’s start with the children who are younger than 12.
So, what you have is, we are already doing, together with the companies, what’s called an age de-escalation and dose — a dose adjustment for younger children. And you do it from 11 to 9, from 9 to 6, from 6 to 2, and then from 6 months to 2 years.
The data have already been very actively collected. We will probably have enough data by the time we get to the early to mid-fall, and then they will be presented to the FDA. Then it becomes a regulatory decision, Judy, where you balance safety for children against the efficacy and the immunogenicity and all the other factors that go into it.
And the data will be there by the middle of the fall. The FDA will have to do what they do well, will have to make a risk/benefit analysis of whatever safety signals, which are good safety…
- 08/23/2021 – vaccine really works in UK and Israel
This is super-interesting. For those of you who aren’t quant geeks, all you need to understand is that the vaccine efficacy data from the UK and Israel are much better than they appear.
For you geeks who want to understand why, let’s first start with the data. Here’s the UK (source):
And here’s Israel (source):
- 08/23/2021 – Pfizer’s vaccine is approved by FDA for people 16 years and older. For children at 12~15 years old might get full approval by Nov or Dec. Pfizer plans to submit a request for booster authorization as an amendment to the full approval. Moderna Inc., MRNA +5.37% whose authorized two-dose shot uses similar mRNA technology as the Pfizer-BioNTech vaccine, has said it is still completing rolling data submissions. Johnson & Johnson, JNJ -0.20% whose shot was authorized in February, has said it plans to file for full approval later this year.
FDA Gives Pfizer-BioNTech Covid-19 Vaccine Full Approval – WSJ
Clearance of the shot, for people 16 years and older, is the agency’s first full approval of a Covid-19 vaccine
The Covid-19 vaccine from Pfizer Inc. PFE +2.95% and partner BioNTech SE BNTX +9.50% received full approval from U.S. regulators Monday, which many public health officials and vaccine experts hope will encourage hesitant populations to get the shot. The Food and Drug Administration’s clearance of the shot for people 16 years and older is the agency’s first full approval of a Covid-19 vaccine.
The vaccine was authorized for children as young as 12 years in May. Pfizer plans to request full approval for that group once it has collected and analyzed six months of safety data from clinical-trial subjects, according to the company.
The vaccine was authorized for children as young as 12 years in May. Pfizer plans to request full approval for that group once it has collected and analyzed six months of safety data from clinical-trial subjects, according to the company.
Pfizer has already submitted data to the FDA showing that a third dose of its vaccine boosts the immune system against the original virus and against the Beta and Delta variants to higher levels than the standard two-dose regimen. Pfizer plans to submit a request for booster authorization as an amendment to the full approval.
Moderna Inc., MRNA +5.37% whose authorized two-dose shot uses similar mRNA technology as the Pfizer-BioNTech vaccine, has said it is still completing rolling data submissions. Johnson & Johnson, JNJ -0.20% whose shot was authorized in February, has said it plans to file for full approval later this year.
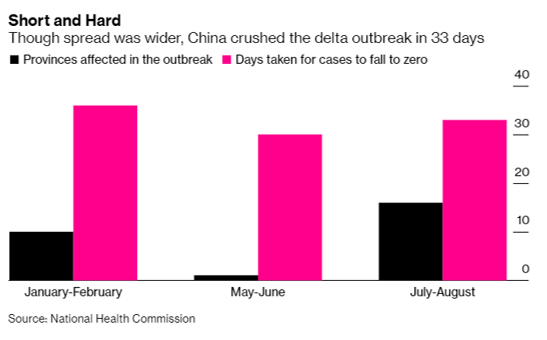
- 08/22/2021 – Again, China spends 33 days to crush the delta outbreak. on the contrary, Australia and US cannot control the outbreak
China Hits Zero Covid Cases With a Month of Draconian Curbs
Bloomberg News
August 22, 2021, 6:32 PM PDT Updated on August 22, 2021, 8:00 PM PDT
Short and Hard
Though spread was wider, China crushed the delta outbreak in 33 days
 Source: National Health Commission
Source: National Health Commission
The blazing spread of the delta variant across the country became the biggest test of China’s Covid control model. Ultimately it penetrated nearly 50 cities across 17 provinces and reintroduced the virus to Wuhan, which had been Covid-free for over a year.
Still, China eliminated the virus in about a month, roughly the same time it took to quell previous outbreaks including one at the start of 2021 that totalled some 2,000 cases. In comparison, cities in Australia have undergone repeated lockdowns, keeping more than half of the country’s 26 million people confined to their homes, without gaining control of the virus. In the U.S., which has never succeeded in containment, relying instead of vaccination, booster shots are slated to roll out next month to shore up protection against its delta resurgence.
- 08/22/2021 – CV infection risks for children are rising significantly in US
More Children Are Hospitalized With Covid-19, and Doctors Fear It Will Get Worse – WSJ
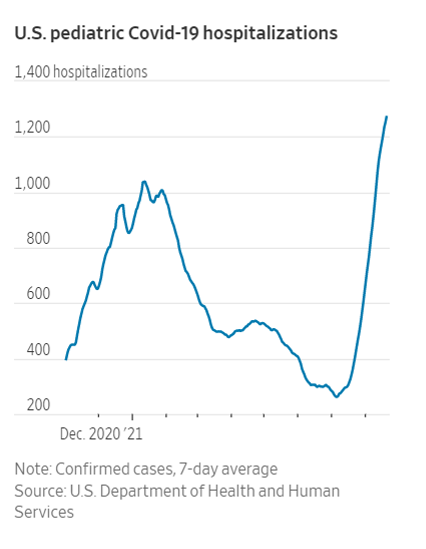
Covid-19’s Delta variant is proliferating world-wide, threatening unvaccinated populations and economic recovery. WSJ breaks down events in key countries to explain why Delta spreads faster than previously detected strains. Composite: Sharon Shi
Oct. 2020 India initiated – Apr, 2021, India peaking – May, 2021, UK – June, 2021 Australia – Aug, 2021, USA
I should always pay attention to these fast changing conditions
- 08/20/2021 – are we approaching the peak of CV cases?
August 20th COVID-19: Cases May be Peaking, by Calculated Risk on 8/20/2021 03:19:00 PM
Some good news: Cases may be peaking, and this is the second consecutive day with over 1 million doses administered!
This data is from the CDC.
The 7-day average hospitalizations is the highest since February 8th.
The 7-day average deaths is the highest since April 1st.
- 08/20/2021 – vaccine might work on Lambda with less efficacy. Only limited study on this. Delta plus is one children of Delta, None of this means the virus itself has suddenly changed. – from MIT Technology Review
According to their results – which have yet to be peer-reviewed – there was a “partial resistance to neutralisation”, however this “is not likely to cause a significant loss of protection against infection” in vaccinated individuals.
But their analysis of the spike proteins on the SARS-CoV-2 Lambda variant showed a two-fold increase in infectivity, which scientists say is due to a particular mutation on the virus called the L452Q mutation.
In a pre-print paper that has not yet been peer-reviewed, researchers found that mRNA vaccines are effective against the Lambda variant. Both the Pfizer and the Moderna coronavirus vaccines used in the UK are mRNA jabs, meaning they contain genetic material that instructs the body’s cells to produce coronavirus spikes, which then provokes an immune response.
The results of this paper suggest that vaccines in current use will remain protective against the Lambda variant.
However, in another pre-print paper, Lambda was found to have mutations that had “the ability to escape from neutralising antibodies elicited by CoronaVac”. CoronaVac is a vaccine being used in several Asian countries, and works by administering an inactive version of the SARS-CoV-2 virus, which then triggers an immune response.
three papers:
https://www.biorxiv.org/content/10.1101/2021.07.02.450959v1
https://www.biorxiv.org/content/10.1101/2021.07.02.450959v1
https://www.medrxiv.org/content/10.1101/2021.06.28.21259673v1
2. Don’t let “delta plus” confuse you. The strain hasn’t learned any new tricks.
It’s worth noting that not all variants with WHO nicknames are equally bad. When the organization gives a new family a name, it also adds a label telling us how worried we should be.
The lowest level is a variant of interest, which means it is worth keeping an eye on; in the middle is a variant of concern, like delta, which has clearly evolved to be more dangerous. Often, variants of interest are given that label because they share a mutation with variants of concern—they’re under surveillance.
The CDC has an additional, more severe category, a variant of high consequence, which has never been given to a family of covid. It’s reserved for potential future strains that might cause serious illness in vaccinated people, fail to show up on commonly used diagnostic tests, or perhaps resist multiple treatments for covid symptoms.
Delta is like a thick branch on that tree—a big family of viruses that share a common ancestor and some of the same mutations, which let them spread between people more quickly. When the big branch grows new twigs, which happens all the time, scientists keep track by using technical names that include numbers and letters. But a new scientific name doesn’t mean those viruses will act any differently from the branch they grew from—and if one of those new branches does start to change its behavior, it gets a new Greek letter, not a “plus.”
(Now is a good time to note that while some of delta’s mutations make it more transmissible, vaccines are still very good at preventing severe illness from every known strain of covid.)
This week, scientists split delta’s “children” into 12 families in order to better track small-scale local changes. None of this means the virus itself has suddenly changed.
all news on Lambda variant
- Lambda COVID variant: All you need to know about the new UK coronavirus strain
- SARS-CoV-2 Lambda Variant Remains Susceptible to Neutralization by mRNA Vaccine-elicited Antibodies and Convalescent Serum
- Infectivity and immune escape of the new SARS-CoV-2 variant of interest Lambda
- Lambda coronavirus variant: What you should know
- Lambda COVID Variant ‘a Potential Threat to Human Society,’ Researchers Say
- Comparison of Neutralizing Antibody Titers Elicited by mRNA and Adenoviral Vector Vaccine against SARS-CoV-2 Variants
- The Lambda COVID variant is in California: 5 things to know
- SARS-CoV-2 Lambda variant exhibits higher infectivity and immune resistance
- Vaccine-resistant lambda variant is in the US
- Here’s Where the Lambda COVID Variant is Currently Spreading
- Study says new Lambda variant could be vaccine-resistant
- 08/19/2021 – Encouraging good news by using CV-19 booster. “These results are highly encouraging. They suggest that the third booster may restore the vaccine efficacy to its original levels,”
Pfizer’s Covid-19 Booster Shot Improves Immunity, Israeli Study Suggests
Early data on those aged 60 and above come as U.S. and other countries plan additional doses to increase protection against Delta variant
TEL AVIV—Early data from Israel suggests a booster shot of Pfizer Inc.’s Covid-19 vaccine can significantly improve immunity in those aged 60 and above, as the U.S. and other countries plan additional doses to increase protection against the highly infectious Delta variant.
Israel was one of the first countries late last month to authorize a third Pfizer dose for the elderly who were fully vaccinated with the recommended two shots, after indications that vaccine protection against severe illness has waned.
The booster shot reduced the risk of infection in the 60-plus age group by 86% and against severe infection by 92%, according to an observational study by Israel’s second largest healthcare provider, Maccabi Health Services, released Wednesday.
“These results are highly encouraging. They suggest that the third booster may restore the vaccine efficacy to its original levels,” said Eran Segal, a computational biologist at the Weizmann Institute of Science and a key adviser to the Israeli government on the pandemic.
- 08/17/2021 – warning from Israel
A grim warning from Israel: Vaccination blunts, but does not defeat Delta
What is clear is that “breakthrough” cases are not the rare events the term implies. As of 15 August, 514 Israelis were hospitalized with severe or critical COVID-19, a 31% increase from just 4 days earlier. Of the 514, 59% were fully vaccinated. Of the vaccinated, 87% were 60 or older. “There are so many breakthrough infections that they dominate and most of the hospitalized patients are actually vaccinated,” says Uri Shalit, a bioinformatician at the Israel Institute of Technology (Technion) who has consulted on COVID-19 for the government. “One of the big stories from Israel [is]: ‘Vaccines work, but not well enough.’”
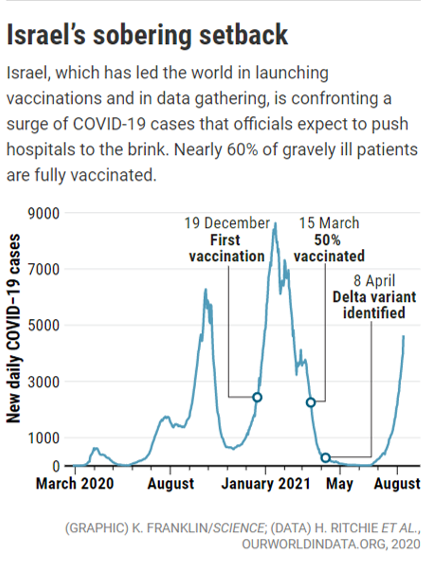
Israel’s HMOs, led by CHS and Maccabi Healthcare Services (MHS), track demographics, comorbidities, and a trove of coronavirus metrics on infections, illnesses, and deaths. “We have rich individual-level data that allows us to provide real-world evidence in near–real time,” Balicer says. (The United Kingdom also compiles a wealth of data. But its vaccination campaign ramped up later than Israel’s, making its current situation less reflective of what the future may portend; and it has used three different vaccines, making its data harder to parse.)
Now, the effects of waning immunity may be beginning to show in Israelis vaccinated in early winter; a preprint published last month by physician Tal Patalon and colleagues at KSM, the research arm of MHS, found that protection from COVID-19 infection during June and July dropped in proportion to the length of time since an individual was vaccinated. People vaccinated in January had a 2.26 times greater risk for a breakthrough infection than those vaccinated in April. (Potential confounders include the fact that the very oldest Israelis, with the weakest immune systems, were vaccinated first.)
- 08/15/2021 – Lambda variant
https://www.forbes.com/sites/williamhaseltine/2021/08/10/it-is-time-to-pay-close-attention-to-the-lambda-variant-now-devastating-south-america/?sh=7d2fbcd14348
https://www.forbes.com/sites/williamhaseltine/2021/08/11/israels-recent-surge-confirms-we-need-a-multimodal-strategy-to-fight-covid-19/?sh=65210c745b6e
https://www.newsweek.com/lambda-covid-variant-potential-threat-society-researchers-1616556
https://news.yahoo.com/vaccine-resistant-lambda-variant-us-193100413.html
https://www.msn.com/en-us/health/medical/is-the-lambda-variant-vaccine-resistant-what-we-know-about-the-covid-19-variant-as-delta-surges/ar-AAMWECk?ocid=uxbndlbing
Arrival of Lambda variant would force lockdown, Health Ministry warns
Officials tell Knesset panel the vaccine is less effective at countering mutation that has rampaged across South America and reached the US
papers,
https://www.biorxiv.org/content/10.1101/2021.07.28.454085v1.full?s=09
https://www.biorxiv.org/content/10.1101/2021.07.19.452771v3.full
https://www.medrxiv.org/content/10.1101/2021.06.28.21259673v1.full
- 08/08/2021 – The pandemic is not coming to an end soon, so I need to be aware of this and response quickly to any sudden changes. the delta variant is “maybe the most contagious virus” ever. Unless we vaccinate everyone in 200 plus countries, there will still be new variants.
The world is nowhere near the end of the pandemic, says famed epidemiologist Larry Brilliant
- The pandemic is not coming to an end soon — given that only a small proportion of the world’s population has been vaccinated, said Larry Brilliant, a well-known epidemiologist.
- Brilliant, who was part of the WHO team that helped eradicate smallpox, said the delta variant is “maybe the most contagious virus” ever.
- The doctor said vaccinated people aged 65 and have a weakened immune system should get a booster shot “right away.”
Dr. Larry Brilliant, an epidemiologist who was part of the World Health Organization’s team that helped eradicate smallpox, said the delta variant is “maybe the most contagious virus” ever.
Unless we vaccinate everyone in 200 plus countries, there will still be new variants.
- 08/05/2021 – new twist in CV-19 development and FDA is working on the full approval of the vaccine
Recent data from Pfizer Inc. and BioNTech SE shows the efficacy of their shot declines about 6% every two months, which suggests boosters may be needed broadly, one of the people said.
Pfizer plans to ask U.S. regulators this month to authorize booster shots of its two-dose vaccine, arguing that a third shot may be needed to protect against the evolving virus.
2. Anthony Fauci: Delta Variant Has ‘Exposed Our Vulnerability’ – The Journal. – WSJ Podcasts
When the FDA finally makes the determination that this is a fully approved vaccine as opposed to an emergency use, you’re going to see people who just on the basis of that alone are going to say, “Okay, now I trust it. Now I’m going to get vaccinated.” But for the (inaudible) people, you’re going to see a much greater confidence in local mandating. What do I mean by that? Big businesses are going to say, “If you want to work for us, you got to get vaccinated.” Cities like New York are saying, “If you want to go in a restaurant, you got to show that you’re vaccinated.” So there’s going to be a lot more confidence in local mandating on the basis of the cover that you’re going to get from the FDA finally fully approving the vaccine. I don’t know when they’re going to do it and I can’t put any pressure on them, and I won’t put any pressure on them. But I hope that it happens in the next few weeks before we come to the end of August.
Anthony Fauci: Delta Variant Has ‘Exposed Our Vulnerability’ – The Journal. – WSJ Podcasts
- 03/29/2021 – serious warning on CV-19 surge. Financial and energy dropped
CDC chief warns U.S. headed for ‘impending doom’ as Covid cases rise again: ‘Right now I’m scared’
- The U.S. is facing “impending doom” as daily Covid-19 cases begin to rebound once again, CDC Director Dr. Rochelle Walensky said during a press briefing.
- The U.S. is recording a weekly average of 63,239 new Covid-19 cases per day, a 16% increase compared with a week ago, according to an analysis of data compiled by Johns Hopkins University.
- When cases rise as they have over the last week or so, Walensky said they often “surge and surge big” shortly thereafter.
- 03/23/2021 – another set back from EU on global economy reopening. Vaccinations are progressing at a glacial pace, Covid-19 cases are spiraling up again and increasingly unpopular governments impose new restrictions weekly.
Europe Despairs as Covid-19 Vaccine Rollout Stalls and Pandemic Grinds On – WSJ
Recurring lockdowns, mounting infections and slow vaccinations take a heavy toll on children and the elderly
Europeans of all ages, from children to grandparents, are becoming exhausted with a crisis that is now entering its second year and whose end seems to be receding beyond the horizon. Vaccinations are progressing at a glacial pace, Covid-19 cases are spiraling up again and increasingly unpopular governments impose new restrictions weekly.
- 03/18/2021 – CV-19 wave is back to EU, could they control it?
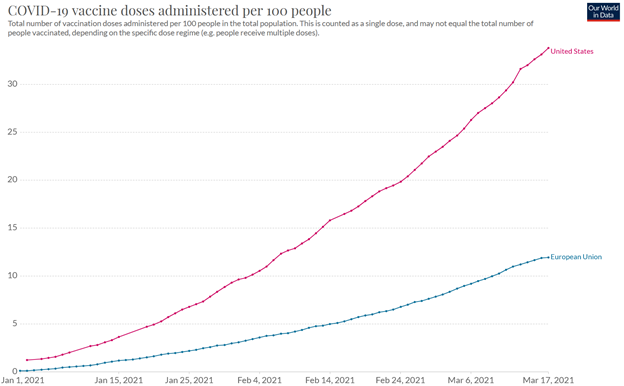
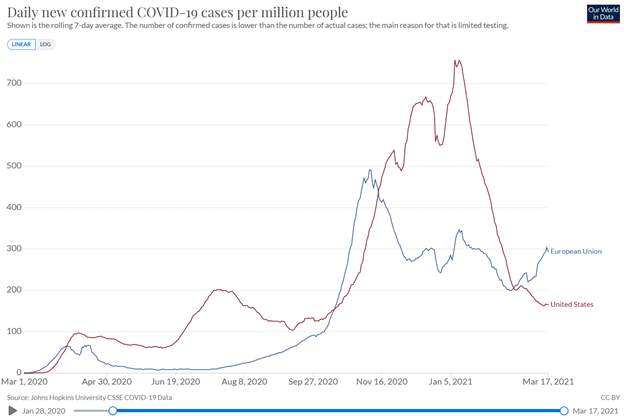
from Tilson’s daily: the European Union is a mess, with vaccinations per capita reaching barely one-third that of the U.S. (source):
|
Not surprisingly, daily new cases are on the rise in the European Union and now exceed the U.S. level on a per-capita basis (source):
|
Here are three articles about the disaster in Europe:
- Italy imposes lockdown measures as cases spike across Europe
- Germany, France, Spain, and Italy are the latest to suspend use of AstraZeneca’s vaccine
- Halting a key vaccine across Europe weakens an already faltering rollout
That said, Europe will figure this out – its incompetence just means the continent will be a few months behind the U.S…
- 02/26/2021 – Democrats are racing to finish the package before March 14,
House Set to Pass $1.9 Trillion Covid Aid Bill – WSJ
Democrats seek alternative approach to lift minimum wage after setback from Senate parliamentarian
The House was on course to narrowly pass a $1.9 trillion Covid-19 aid bill Friday as Democrats moved to pull together an alternative plan to lift workers’ wages after the Senate parliamentarian ruled out a minimum-wage increase.
Democrats are racing to finish the package before March 14, when certain types of federal unemployment assistance are set to expire.
- 02/22/2021 – House might pass $1.9T bill this week. Immediate boost for market. Should I play short term LEAPs?
House panel advances $1.9 trillion Covid relief bill as Democrats move toward passage this week
- The House Budget Committee voted to advance the $1.9 trillion coronavirus relief package.
- The move sets the stage for the House to pass the bill, possibly later this week.
- 02/17/2021 – House will prepare to work through Feb. 26 and into the ensuing weekend in order to pass the relief bill. So be aware of this upcoming possible catalyst.
Democrats focus on passing Covid relief bill after Trump’s acquittal
- Democrats are devoting their full attention to passing a coronavirus relief package after the conclusion of former President Donald Trump’s second impeachment trial.
- The House aims to pass the aid bill, which includes stimulus checks, an extension of unemployment programs and vaccine distribution funds, before the end of the month.
- Democrats are using budget reconciliation to pass the bill without Republican votes but will still face some challenges in getting the plan through Congress.
On Tuesday, House Majority Leader Steny Hoyer, D-Md., told lawmakers to prepare to work through Feb. 26 and into the ensuing weekend in order to pass the relief bill. House Democratic Caucus Chair Hakeem Jeffries will hold calls this week with members of committees putting together the legislation, NBC News reported.
- 02/16/2021 – Good to accelerate the vaccination process
- The Biden administration will send out 13.5 million doses of Covid-19 vaccine per week, up from 11 million last week, White House Press Secretary Jen Psaki said Tuesday.
- The White House will also double the number of doses sent directly to retail pharmacies, Psaki said, up from 1 million last week.
- More than 52.8 million doses have been administered as of Sunday, according to data from the Centers for Disease Control and Prevention, out of 70 million doses delivered to states.
The Biden administration is increasing the number of Covid-19 vaccine doses shipped to states weekly, sending out 13.5 million doses this week and doubling the number going to retail pharmacies, White House press secretary Jen Psaki said Tuesday.
As of last week, the administration was sending out 11 million doses to states every week. Overall, Psaki noted, the administration has increased the number of doses shipped weekly to states by 57% since President Joe Biden was inaugurated Jan. 20.
“It may take until June, July and August to finally get everybody vaccinated,” he said on CNN. “So when you hear about how long it’s going to take to get the overwhelming proportion of the population vaccinated, I don’t think anybody disagrees that that’s going to be well to the end of the summer and we get into the early fall.”
- 02/14/2021 – The coming week might be quiet for stimulus plan, we will more update the week after next. Should I prepare for short term spike?
Here’s how Trump’s acquittal could affect Biden’s stimulus checks
House Speaker Nancy Pelosi told reporters on Thursday she hopes the package is approved “by the end of February” as the March 14 expiration on unemployment aid looms
Following the conclusion of former president Donald Trump’s impeachment trial, the House and Senate are schedule to take a short recess this upcoming week, delaying Congress’ effort to deliver a third round of stimulus checks and other much-needed relief to the American people.
Lawmakers will reconvene on February 22, when they will iron out the outstanding details of President Biden’s $1.9 trillion proposal.
- 02/12/2021 – CDC and White House are pushing to reopen schools safely as soon as possible – good for economy to reboot
CDC Presses K-12 Schools to Reopen
Public-health agency issues new guidelines for schools to reopen safely
- 02/11/2021 – the $1.9T CV relief will be drafted by Friday, and voted in House by the end of Feb., might be signed into law before March 14, when key unemployment programs expire. These are critical timelines to follow.
Pelosi expects Covid relief will be signed into law before unemployment programs expire
- House Speaker Nancy Pelosi said the House hopes to pass its coronavirus relief bill by the end of the month and see it signed into law before March 14, when key unemployment programs expire.
- Senate Majority Leader Chuck Schumer said the impeachment trial of former President Donald Trump will not delay the chamber’s passage of the bill.
- Schumer appeared to endorse the House proposal for stimulus check eligibility, and said he is working to include a $15 per hour minimum wage in the legislation.
The House hopes to approve its $1.9 trillion aid plan “by the end of February so we can send it to the president’s desk before unemployment benefits expire” on March 14, the California Democrat told reporters.
Nine House committees this week started to write and advance their portions of the relief bill, which Democrats are expected to pass through budget reconciliation without Republican votes. Pelosi said she expects the panels crafting the legislation will finish their work this week.
The Budget Committee will then combine the policies. Once the completed bill goes through the Rules Committee, the full House can vote on it.
The legislation faces more challenges in the Senate than in the House. In an evenly split chamber, a single Democratic defection would stop the bill’s approval.
- 02/09/2021 – excellent news on pandemic
From Tilson’s daily: The news regarding the pandemic continues to be excellent…
Here are the latest data for testing, cases (down 75% since the peak a month ago), hospitalizations (down 39%), and deaths (down 70%, though the seven-day average is only down 12%) (source):
|
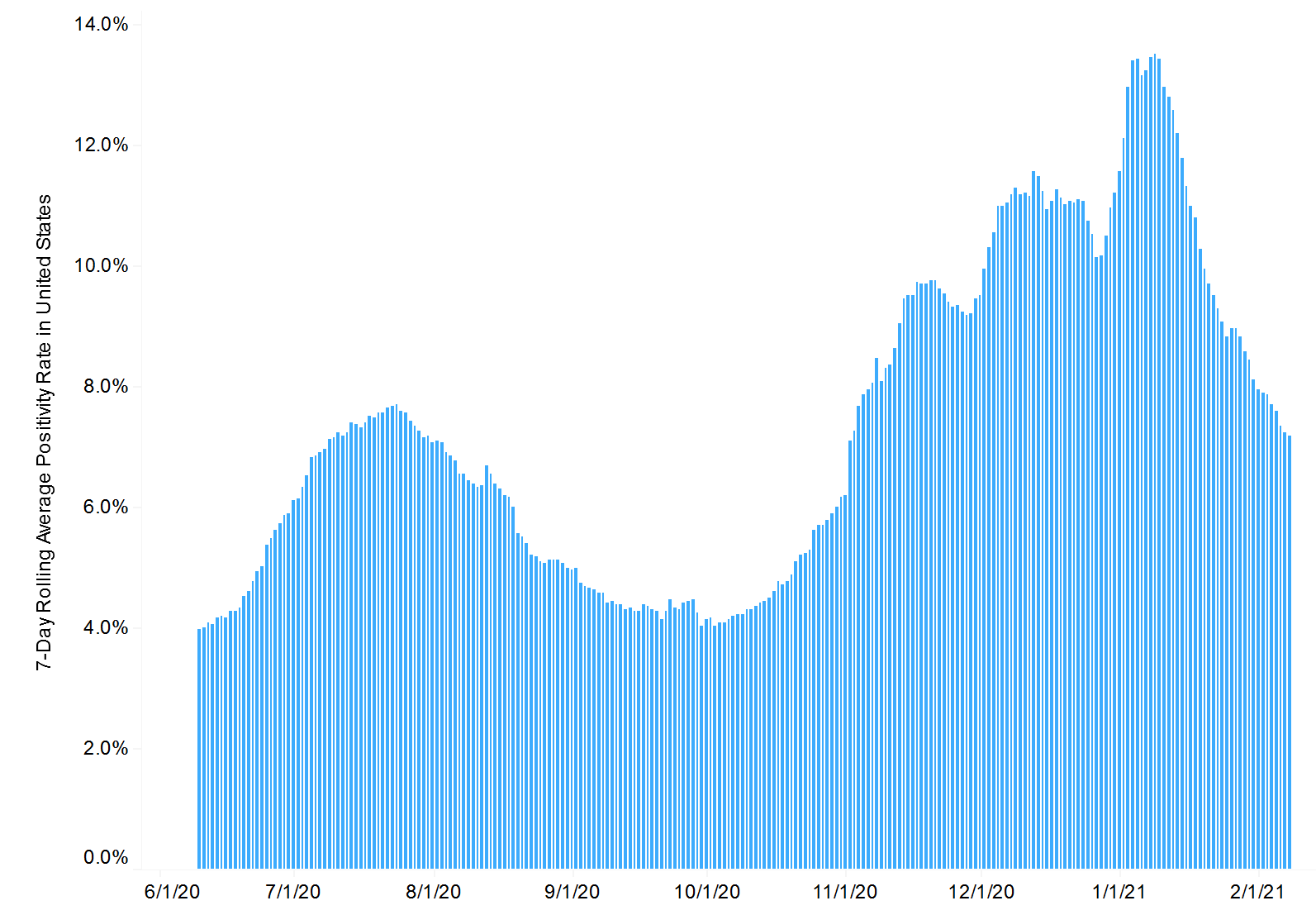
The positivity rate is also falling rapidly – down by nearly half from 13.6% a month ago to 7.2% today (source):
|
(If you click the source links above, you can see all of this data by state, plus here are the data for New York City.)
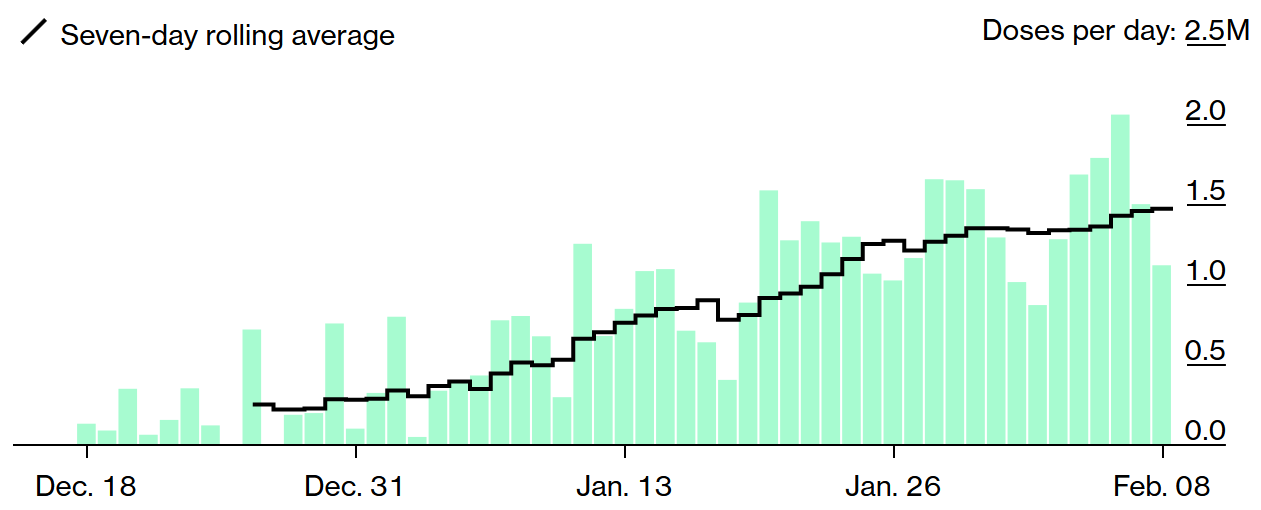
Lastly, as I noted in yesterday’s e-mail, over the past seven days we’ve averaged 1.5 million doses of the vaccine administered per day… and on Saturday, the U.S. had its first day of more than 2 million doses (source)!
|
As a result, we have administered 13.1 doses per 100 people (since some people have received two doses, I estimate that 10% of Americans have received at least one dose of a vaccine) (source):
|
We continue to be among the world leaders in vaccinating our population, trailing only the U.K. among major countries (source):
- 02/08/2021 – be cautious about the bumpy road of recovery
Biden says it will be difficult to achieve Covid herd immunity before summer’s end
- President Biden made his comment in a CBS interview that aired before Sunday’s Super Bowl.
- “The idea that this can be done and we can get to herd immunity much before the end of this summer is very difficult,” Biden said.
- The comment came after journalist Norah O’Donnell said that at the current rate, it would take almost a year to vaccinate enough Americans to achieve herd immunity.
- 02/05/2021 – the final stimulus package is planned to be completed before 03/14. So watch out this time. Plan our a LEAP expiration after this date.
Houses passes budget resolution for coronavirus relief after Senate’s marathon session
Now the House will get to work on drafting details of the coronavirus relief package
The House of Representatives on Friday passed an updated budget resolution sent over from the Senate that paves the way for Democrats to push through President Biden’s $1.9 trillion coronavirus relief package without needing any GOP support.
The vote was 219-209 to adopt the budget framework that the Senate approved early Friday morning thanks to a tie-breaking vote cast by Vice President Kamala Harris.
The measure unlocks the process for lawmakers to now draft a final coronavirus relief bill under the budget reconciliation rules that would let Democrats avoid a GOP filibuster and pass the major stimulus legislation on their own as long as their caucus remains united.
House Speaker Nancy Pelosi, D-Calif., and Democratic leaders huddled with Biden at the White House Friday afternoon. Pressed on whether they’d finalize the major aid package before March 14 when the current unemployment insurance benefits expire, Pelosi was confident about meeting the deadline.
“Absolutely,” Pelosi said outside the White House. “Without any question. Before then.”
- 02/05/2021 – J&J’s vaccine’s approval this month might be another catalyst for stock market. We might have 300 mil dose more from J&J. Great to fight the pandemic.
Johnson & Johnson requests emergency authorization from FDA for Covid vaccine
- Johnson & Johnson applied for an emergency use authorization from the FDA for its coronavirus vaccine.
- The company released data last week showing it was about 66% effective in protecting against the virus.
- If J&J’s application is approved, it would be the third Covid-19 vaccine authorized for emergency use in the U.S. behind those developed by Pfizer-BioNTech and Moderna.
Johnson & Johnson applied for an emergency use authorization from the Food and Drug Administration for its coronavirus vaccine after releasing data last week showing it was about 66% effective in protecting against the virus.
U.S. officials and Wall Street analysts are eagerly anticipating the authorization of J&J’s vaccine, which could happen as early as this month.
J&J said on Jan. 29 that its vaccine was 66% effective overall in protecting against Covid-19. The vaccine, however, appeared to be less potent against other variants. The level of protection was just 57% in South Africa, where a new, highly contagious strain called B.1.351 is rapidly spreading. South Carolina officials detected the first-known U.S. case of that strain last month.
Dr. Anthony Fauci, the nation’s leading infectious disease expert, said the most crucial finding of the J&J data was the vaccine appeared to be 85% effective in preventing severe disease.
“The most important thing, more important than whether you prevent someone from getting aches and a sore throat, is preventing people” from getting severe disease, the director of the National Institute of Allergy and Infectious Diseases said on a call with reporters on Jan. 29. “That will alleviate so much of the stress and human suffering and death in this epidemic.”
J&J has said it plans to ship the vaccine at 36 to 46 degrees Fahrenheit. By comparison, Pfizer’s vaccine needs to be stored in ultra-cold freezers that keep it between negative 112 and negative 76 degrees Fahrenheit. Moderna’s vaccine needs to be shipped at between negative 13 and 5 degrees Fahrenheit.
The Department of Health and Human Services announced in August that it reached a deal with Janssen, J&J’s pharmaceutical subsidiary, worth approximately $1 billion for 100 million doses of its vaccine. The deal gives the federal government the option to order an additional 200 million doses, according to the announcement.
- 02/02/2021 – another good news on vaccine – plan to vaccinate 60% of population in Russia, other countries are following suit
Russian Covid-19 Vaccine Was Highly Effective in Trial, Study Finds, Boosting Moscow’s Rollout Ambitions
Sputnik V shot achieved 91.6% efficacy against coronavirus symptoms, says paper in U.K.’s Lancet
MOSCOW—Russia’s homegrown Sputnik V vaccine showed high levels of efficacy and safety in a peer-reviewed study released Tuesday, a potential boost for the Kremlin’s aim to promote the Covid-19 shot abroad and curb the pandemic at home.
The findings, from a preliminary analysis of a large-scale clinical trial published in the British medical journal the Lancet, demonstrated that the two-shot vaccine was 91.6% effective against symptomatic Covid-19 and offered complete protection against severe cases. There were no serious side effects, the paper said. The vaccine was also found to be similarly safe and effective in elderly people.
The results published on Tuesday were based on an interim analysis of a Phase 3 trial of nearly 20,000 participants, three-quarters of whom received the vaccine while the rest received a placebo. The analysis was based on a total of 78 confirmed Covid-19 cases, 62 of which were identified in the placebo group and 16 in the vaccine group. The clinical trial, totaling 40,000 volunteers, is ongoing.
Russia—the world’s fourth worst-hit country with nearly four million cases—has also banked on Sputnik V to avoid new costly lockdowns as authorities plan to vaccinate 60% of the domestic population by the end of the year.
The shot, which was approved by Russian authorities in August before undergoing large-scale clinical trials, has stirred questions in light of its fast-tracked development and lack of published trial data. So far, Sputnik V has been administered to more than two million people world-wide, including in Argentina, Serbia and Algeria, according to Russian authorities.
- 02/02/2021 – Using the reconciliation process to approve Biden’s $1.9T stimulus plan will likely take several weeks. The House is scheduled to vote on a budget resolution this week that will include reconciliation instructions that will allow Congress to draft and ultimately pass a coronavirus bill with only a simple majority in the House and Senate. As Biden continues to confer with Senate Republicans, all eyes on the Democratic side are on Sen. Joe Manchin (D-W.Va.), who is square in the middle in the battle between GOP centrists and the size of the future stimulus package. – so there is still not certain the plan will be passed or not
Psaki, referring to the dual crises of a pandemic and high unemployment, repeated that Biden “will not settle for a package that fails to meet the moment.” The comments came as Speaker Nancy Pelosi (D-Calif.) and Senate Majority Leader Charles Schumer (D-N.Y.) announced they would work to pass a budget this week, instructing the relevant committees to write a $1.9 trillion stimulus bill mirroring Biden’s ideas. This sets the stage for a complex strategy that requires only 50 votes for enactment in the evenly divided Senate. As vice president, Harris would be the tie-breaker.
As The Hill’s Morgan Chalfant writes, Democrats argue that Biden’s approach will keep the door open to a bipartisan compromise. However, it ensures that a bill can be passed before certain unemployment benefits expire in mid-March. Using the reconciliation process will likely take several weeks.
As Biden continues to confer with Senate Republicans, all eyes on the Democratic side are on Sen. Joe Manchin (D-W.Va.), who is square in the middle in the battle between GOP centrists and the size of the future stimulus package.
Many Democrats have dismissed the Republicans’ $618 billion relief proposal as a fraction of what is needed. However, Manchin has yet to say whether he supports the effort by Democrats to move a proposal via budget reconciliation with a simple majority. His hesitation also comes as the administration ups the pressure on him, with Harris telling a local TV station in the state that the full package is needed because 1 in 7 families in the state are hungry (The Hill).
GOP senators unveil $618 billion coronavirus proposal ahead of Biden meeting
White House press secretary Jen Psaki indicated on Monday that Biden would not be cutting a deal with Republicans during the meeting.
“What this meeting is not is a forum for the president to make or accept an offer,” Psaki said.
The House is scheduled to vote on a budget resolution this week that will include reconciliation instructions that will allow Congress to draft and ultimately pass a coronavirus bill with only a simple majority in the House and Senate.
Going it alone will test Democratic unity just weeks into Biden’s administration. Democrats have a slim majority in the House and the 50-member entire Senate Democratic caucus would need to support both the budget resolution and the subsequent coronavirus legislation in order to pass it without GOP support.
Democrats are split over whether or not including a $15 minimum wage increase complies with arcane Senate rules that govern what can, and cannot, pass through reconciliation. A stand-alone bill from Sen. Bernie Sanders (I-Vt.) only has the support of an additional 37 senators.
Sen. Joe Manchin (D-W.Va.) refused to tell reporters late last week if he would vote for the budget resolution if it’s used to set up a Democratic-only coronavirus bill.
“We’re gonna try to make Joe Biden successful. I don’t understand why y’all don’t understand what I’m saying,” he said amid several questions about his vote.
- 01/31/2021 – a large portion of the world will still be battling the pandemic and its economic effects well into 2022 or beyond – I need to prepare for the long haul
Vaccination Delays Put Global Rebound at Risk
Slipping timetables for inoculation campaigns mean return to normal could get pushed back for many countries
Timetables for vaccinating enough people to effectively curb Covid-19 are slipping in many countries, raising fears that a large portion of the world will still be battling the pandemic and its economic effects well into 2022 or beyond.
While the U.S. and some other mostly small countries are making progress toward vaccinating most of their populations by late summer, health experts and economists are concluding that much of the planet—including parts of Europe, Asia and Latin America—face a longer slog.
China also faces challenges. Although it has started inoculations using homegrown vaccines, without providing a firm timeline for reaching herd immunity, approvals and production arrangements have come more slowly than anticipated, according to Trivium China, a consultancy.
In one sign of the difficulties, the Beijing government’s talent office said that vaccine producer Sinovac is struggling to hire new staff.
“The main issue is production volume,” said Guo Wei, deputy secretary general of the health-care logistics association at the government-backed China Federation of Logistics and Purchasing, in an interview. He said that based on production estimates by China’s vaccine makers, the country wouldn’t be able to reach herd immunity this year.
Trivium estimates that a total of 850 million doses is the high end of what is possible for China this year, while administering at least 1.68 billion doses would be considered full inoculation. The Economist Intelligence Unit doesn’t rule out some major Chinese cities reaching herd immunity this year but estimates that the country as a whole likely won’t be able to reach it until late 2022.
- 01/26/2021 – Great to have more vaccines
Biden administration aims to have enough vaccine for most Americans by summertime
WASHINGTON (Reuters) – The United States aims to acquire an additional 200 million doses of COVID-19 vaccines, President Joe Biden said on Tuesday, enough to inoculate most Americans by summertime, as he races to curb a pandemic he warned could still get worse.
Biden’s administration will purchase 100 million doses each of the vaccines made by Pfizer Inc and BioNTech, and Moderna Inc, increasing the overall total doses to 600 million, with delivery expected by summer.
The previous purchase target was 400 million doses.
Each vaccine requires two doses per person to be fully effective, suggesting the new purchases would build up enough of a stockpile to inoculate most of the country’s 331 million people. The vaccines are not approved for use by most children.
On Monday, Biden said he believed it was possible to have 150 million doses of the vaccine administered in his first 100 days in office, an aspiration his press secretary, Jen Psaki, said was not an official adjustment of the current target of 100 million doses over that same time period.
Biden Says U.S. Could Be ‘Heading Toward Herd Immunity’ by Summer
President Biden said Monday his target of administering 100 million Covid-19 vaccines in his first 100 days in office might rise to 150 million.
Addressing reporters at the White House, Mr. Biden said he was hopeful the U.S. could soon reach an average of 1.5 million vaccines per day, as opposed to the current pace of 1 million a day. He also said he was optimistic that by this spring, any American who wants a coronavirus vaccine should be able to get one.
“It’s going to be a logistical challenge that exceeds anything we’ve ever tried in this country, but I think we can do that,” Mr. Biden said. “I feel confident that by summer, we’re going to be well on our way to heading toward herd immunity and increasing the access for people who aren’t on the list, all the way going down to children.”
- 01/22/2021 – Implication of virus mutation means new version of vaccine might be needed and new phase 3 test might be needed. Virus mutation has much greater chance of obliterating the efficacy of a monoclonal antibody such as monoclonal antibodies from Eli Lilly (LLY) and Regeneron (REGN).
New Covid-19 Strains May Make Vaccines Less Effective, Fauci Warns
In his first White House press briefing of the Biden administration, Dr. Anthony Fauci warned that new strains of the virus that causes Covid-19 now in circulation could make the currently available vaccines and antibody therapies less effective.
“We’re paying very, very careful attention to this, and we take it very seriously,” Fauci said.
Fauci was careful not to sound a general alarm. “Right now, from the reports we have—literally, as of today—it appears that the vaccines will still be effective against [the new strains], with the caveat in mind you want to pay close attention to it,” Fauci said Thursday.
Fauci said that there is greater concern for the effectiveness of the monoclonal antibody drugs that are being used to treat and, in clinical trials, to prevent, Covid-19 infections. “Since monoclonal antibodies bind to a very specific part of the virus, when there’s a mutation there, it has much greater chance of obliterating the efficacy of a monoclonal antibody,” Fauci said. “And we’re seeing in the much more concerning mutations that are in South Africa—and in some respects, Brazil, which is similar to South Africa—that it is having an effect on the monoclonal antibodies.”
New data shows that Covid vaccines may not be as effective in guarding against new, more contagious strains of the virus, Fauci also said Thursday. Some variants identified in the U.K., South Africa and Brazil appear to be more transmissible but not necessarily more deadly. (CNBC)
The Food and Drug Administration has given emergency use authorizations to Covid-19 monoclonal antibodies from Eli Lilly (LLY) and Regeneron (REGN). Lilly reported Thursday that its antibody appeared to have been able to reduce the risk of nursing home residents and staff coming down with symptomatic Covid-19 infections. And Regeneron, earlier this month, announced a deal with the U.S. government worth up to $2.6 billion for as many as 1.25 million doses of its Covid-19 antibody.
Fauci also said that there are plans to update the vaccines if necessary. Last week, BioNTech’s CEO said at investor conference that his company could have an updated version of its vaccine available within six weeks. Pfizer’s chief scientific officer, meanwhile, told Barron’s that the company should “aspire to” getting a new version of the vaccine out in three to four months, if it were needed, with time for preclinical testing and a human trial.
It will be up to regulators to determine how much human testing would be needed for a new version of the vaccine.
- 01/14/2021 – Biden’s two pronged plan (rescue plan and recovery plan) is very ambitious. If vaccines can be distributed faster, then it will be great. Really want to see the recovery plan (infrastructure and climate control).
Biden Proposes $1.9 Trillion Covid-19 Relief Package
President-elect urges Congress to back $1,400 per-person direct payments and asks for funding for testing and vaccine distribution
President-elect Joe Biden is calling for a $1.9 trillion Covid-19 relief plan to help Americans weather the economic shock of the pandemic and pump more money into testing and vaccine distribution.
Mr. Biden said that in February he would outline a second proposal focused on economic recovery that would also use jobs and infrastructure as a tool to combat climate change
Last year, lawmakers reached a stalemate when Republicans resisted aid for state and local governments and Democrats objected to a liability shield for businesses. The result was a postelection deal of about $900 billion, which Democrats described as a down payment.
Now, because they won both Senate seats in Georgia this month, Democrats could use special fast-track rules known as budget reconciliation to advance a more partisan bill, which would require just 51 votes in the Senate. If the 50 Democrats remain united, they could tap Vice President-elect Kamala Harris to cast the tiebreaking vote. But that path comes with restrictions, including a limited number of times Democrats can use it this year and rules that confine reconciliation bills to tax and fiscal matters rather than broader policies.
If manufacturing projections previously put forth by companies hold up, Mr. Biden’s pledge to administer 100 million doses of Covid-19 vaccines during the first 100 days of his presidency should be possible, according to manufacturing and supply chain experts.
- 01/12/2021 – Good for China economy to recover confidently: Despite having one of the lowest efficacy rates for any new coronavirus vaccine, CoronaVac is still more effective than some flu vaccines and can be stored cheaply in an ordinary refrigerator, making it a viable option for developing countries, public health experts said
Chinese Covid-19 Vaccine Is Far Less Effective Than Initially Touted in Brazil
Late-state testing results of Sinovac’s shot were almost 30 percentage points lower than previously announced, as concerns grow over the study’s transparency
Brazilian researchers testing China’s Sinovac Vaccine said Tuesday that full data showed it to be 50.38% effective against Covid-19 in late-stage trials, almost 30 percentage points lower than previously announced, as concerns grow over the study’s transparency.
The São Paulo-based Butantan Institute said last week that late-stage trials had shown the CoronaVac vaccine to be between 78% and 100% effective, offering total protection against severe cases of the disease.
However, after rising pressure from scientists, some of whom accused the trial’s organizers of misleading the public, Butantan said those rates only included volunteers who suffered mild to severe cases of Covid-19. When data from all volunteers is considered, including those who contracted “very mild” cases of Covid-19 and required no medical assistance, the total efficacy rate falls to 50.38%, Butantan said Tuesday.
Despite having one of the lowest efficacy rates for any new coronavirus vaccine, CoronaVac is still more effective than some flu vaccines and can be stored cheaply in an ordinary refrigerator, making it a viable option for developing countries, public health experts said.
However, the piecemeal and unorthodox announcement of CoronaVac’s efficacy rate, which was first scheduled to be announced last month, risks damaging the credibility of a vaccine that many Brazilians are already reluctant to take, doctors said.
- 01/07/2021 – 2 years protection from disease is great, if it is proved to be true
Moderna CEO says COVID-19 vaccine likely to protect for ‘couple years’
Moderna’s CEO made a bold prediction Thursday about the amount of immunity his company’s COVID-19 vaccine may provide, suggesting that the recently approved vaccine could offer protection of up to a couple of years.
“My expectation is that prevention of disease by these vaccines will last quite long, maybe prevention of infection to the level we’re seeing, maybe shorter-lasting, maybe lasts three, four, six months,” Slaoui previously said on “Andrea Mitchell Reports.” “When prevention of disease, in my humble opinion as an expert, is probably going to last a year or two years, three years.”